
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 06 September 2019
Sec. Crop Biology and Sustainability
Volume 3 - 2019 | https://doi.org/10.3389/fsufs.2019.00064
This article is part of the Research TopicProceedings of Olivebioteq 2018 – Olive Management, Biotechnology and Authenticity of Olive ProductsView all 22 articles
Non-crop cultivated plants can provide agriculture with ecosystem services, such as biological pest control and, a sound knowledge of the relationships between these plants and arthropod communities is important. Given its entomophilous characteristics, Dittrichia viscosa, a plant commonly found in the Mediterranean region, could potentially be used in integrated pest management systems. The aim of this study is to investigate arthropofauna associated with D. viscosa in olive groves during its pre-flowering, flowering and post-flowering stages and to determine the possible relationships between different groups of arthropods. Using vacuum-sampling, the study was carried out on D. viscosa plants bordering and inside olive groves. The plants produced new leaves in April and flowered between August and October. Miridae, Aphididae, Hymenoptera parasitoids, Formicidae, Araneae, and Aleyrodidae were the most abundant groups of arthropods collected during the pre-flowering and flowering stages. Plant phenology differentially influenced the arthropod populations of the different groups, with the Aleyrodidae family found to be more abundant during the pre-flowering stage, while Hymenoptera parasitoids were more numerous during the flowering stage. During the post-flowering stage, the number of arthropods captured was very low. Numerous correlations between and within the different functional groups were observed throughout the life cycle of D. viscosa. Our results clearly show that D. viscosa plants in olive groves have great potential as a reservoir of different predators and Hymenoptera parasitoids and that these olive groves were not attacked by any D. viscosa-related phytophages.
The intensification of agriculture has led to a simplification of the landscape and a reduction in biodiversity, which have affected various ecosystem services, such as natural pest control and pollination (Zhang et al., 2007). The agroecological strategy of habitat management could help to reverse this situation by enhancing conservation biological control, as the presence of semi-natural habitats increases landscape diversity and supplies resources (pollen, nectar, alternative hosts, refuge, and oviposition sites) to natural enemies and pollinators which provide these ecosystem services (Landis et al., 2000; Bianchi et al., 2006; Holland et al., 2016, 2017). However, as the effect of landscape and vegetation on arthropod populations (pests and natural enemies) is based on complex mechanisms, the conditions that contribute to increasing and reducing biological control need to be investigated. It is also crucial to determine how key plant species are managed (Simon et al., 2010; Carrié et al., 2012; Chaplin-Kramer et al., 2013; Miñarro and Prida, 2013). To do this, it is necessary to explore the relationships between available resources in each type of habitat and its associated arthropod community, the spatial-temporal distribution of resources in the landscape and interactions with other factors that play a role in pest regulation (Holland et al., 2016).
Dittrichia viscosa (L.) Greuter (Asterales: Asteraceae) is a plant of considerable interest, given its distribution throughout the Mediterranean region, its adaptation to a wide range of stress conditions, and its various uses including phytoremediation, as well as its role as a bioaccumulator and bioindicator (Parolin et al., 2014). In addition, given its entomophilous character, it has great potential for use in the integrated pest management of Mediterranean agroecosystems (Parolin et al., 2014). Indeed, D. viscosa has been proven to play an outstanding role in maintaining and expanding predatory mirid populations in different agroecosystems (Alomar et al., 2002; Perdikis et al., 2007; Lambion, 2011; Lykouressis et al., 2012) and as a reservoir of aphid parasitoids (Kavallieratos et al., 2002) and phytoseiid mites (Tixier et al., 2000). However, it is also worth noting that D. viscosa can boost the presence of phytophages, such as whiteflies (Homoptera: Aleyrodidae) (Parolin et al., 2013) and can act as a reservoir of tomato infectious chlorosis viruses (Orfanidou et al., 2016).
In olive groves, numerous studies are being carried out on the role played by different types of vegetation in biological pest control and other regulatory ecosystem services, such as fertility, erosion and pollination (Villa Serrano, 2016; Alcántara et al., 2017; Paredes et al., 2017; Porcel et al., 2017; Gómez et al., 2018). The plant species D. viscosa associated with olive groves, is of considerable interest in relation to its role in olive pest control, as its flowers are attacked by the gall-producing Myopites stylatus (Fabricius, 1974) (Diptera: Tephritidae). Its larvae are parasitized by Eupelmus urozonus (Hymenoptera: Eupelmidae), which, in turn, parasitizes the olive fly Bactrocera oleae (Rossi, 1790) (Diptera: Tephritidae), one of the principal olive pests (Warlop, 2006; Franco-Micán et al., 2010; Mota et al., 2011). It is also important to note that the flowering period of D. viscosa lasts from September to October (Parolin et al., 2014), when floral resources in olive groves are scarce and crop vegetation cover has been eliminated due to soil management requirements (Alcántara et al., 2017). This explains why, among native Mediterranean plants, D. viscosa is considered a potential source of food for natural enemies of olive pests (Nave et al., 2017). Its floral architecture has been found to facilitate access to pollen and nectar for four of the principal parasitoids of Prays oleae (Bernard, 1788) (Lepidoptera: Plutellidae), another important olive pest. However, it also prevents access to adults of this phytophage and Chrysoperla carnea, one of its most notable predators (Nave et al., 2016).
Before floral resources are introduced into an agroecosystem to improve or expand ecosystem services, such as biological control, in-depth entomological and agronomic studies need to be carried out (Araj and Wratten, 2015), as the arthropod community can respond in different ways to environmental factors at the local and spatial level, as previously observed in the case of spiders in olive groves (Picchi et al., 2016).
Thus, the objective of this study is to investigate arthropofauna associated with D. viscosa present in olive groves during its pre-flowering, flowering and post-flowering stages and to determine the possible relationships between different groups of arthropods.
The study was carried out in two olive (Olea europaea L.) groves (Granada 37°10′34″N; 3°34′51″W; 880 m and Jaén 37°40′49″N; 3°48′3″W; 872 m) in Andalusia (Spain). The olive variety was “Picual,” with a planting layout of 10 × 10 m. D. viscosa natural plants can be found in these olive groves, some bordering and others inside the crop. In the latter case, there are also cultivated D. viscosa plants between the rows of olive trees. As in the case of ditches and trenches in roads, this plant, which is attracted by unstable and collapsed lateral walls, has colonized most of the gullies in the olive groves (Simões et al., 2013; Parolin et al., 2014).
The natural plants bordering the olive groves are surrounded by olive trees, grazing land, such as Daucus carota L., Convolvulus arvensis L., Taraxacum sp. F. H. Wigg., and Foeniculum vulgare Mill., and forest mainly composed of Pinus sp. L., Quercus coccifera L., and Quercus rotundifolia Lam. The cultivated D. viscosa plants located inside the crop are placed one meter apart and are surrounded by olive trees and others plant species, such as Capparis spinosa L., Daucus carota L., Cichorium intybus L., Convolvulus arvensis L., Psoralea bituminosa L., Bromus rubens L., and Foeniculum vulgare Mill. The distance between the natural plants, which are in gullies, varies from one to three meters and are surrounded by olive trees and different herbaceous and shrub species, such as Rubus idaeus L., Spartium junceum L., Asparragus acutifolius L., Smilax aspera L., Aristolochia baetica L., Cynodon dactylon L., and Carduus sp. L.
Both olive groves have a meso-Mediterranean climate, with an average temperatures ranging from 15 to 18°C and an average rainfall of between 477 and 560 mm (Valle Tendero et al., 2005).
The study was carried out between June and December 2014. In order to analyze the arthropods in the different phenological stages of D. viscosa, sampling was carried out in June during the pre-flowering stage, in September during the flowering stage and in November during the post-flowering stage in the three areas studied: bordering (natural plants) and inside the olive grove (cultivated and natural plants). In each area, five sites were sampled, and the plants were vacuum-sampled for 40 s with the aid of an entomological aspirator (Modified CDC Backpack Aspirator Model 1412, John W. Hock Co., Gainsville, FL, USA). After vacuuming, the samples were labeled and cold-stored to avoid any interactions between the arthropofauna captured before going to the laboratory where they were stored at −20°C.
Under a stereomicroscope (Nikon SMZ800 Model CP-S, Nikon Co., Tokyo, Japan), the samples were cleaned and the arthropods were separated from vegetal material, which were then conserved in 70% alcohol. All the material was identified using Hymenoptera of the World (Goulet and Huber, 1993), Heteroptera Families of the Iberian Peninsula (Mata and Goula, 2011) and Bases para un Curso Práctico de Entomología (Barrientos, 1988) as keys. We also used molecular analysis to identify the mirid species Macrolophus (Castañé et al., 2013).
The phenological states of D. viscosa were noted at each sampling and subsequent visits.
All analyses were carried out using the R program version 3.5.0 (R Development Core Team, 2017). Analysis began with a data exploration (Zuur et al., 2010). We analyzed differences in the abundance of the majority taxonomic groups of phytophages (Aphididae and Aleyrodidae), predators (Araneae, Formicidae, Miridae) and Hymenoptera parasitoids according to their location (bordering or inside the olive grove) and the phenological stage of D. viscosa (pre-flowering, flowering, and post-flowering). In the case of plants situated inside the olive groves, we needed to pinpoint any differences between cultivated and natural D. viscosa plants. Depending on whether or not abundance data followed a normal distribution pattern, we opted for the Least Significant Difference test (Fisher-LSD) or the Kruskal-Wallis test, with a Bonferroni adjustment in both cases, using the “agricolae” software package (De Mendiburu, 2017). Additionally, we performed Pearson correlations with a confidence level of 95% (α = 0.05) between the taxonomic groups in all cases.
Following the winter rest period, with the appearance of new leaves, D. viscosa emerged in early April, continued to grow until mid-June and stopped growing with the arrival of summer and drought conditions. The plants began to produce flower buds at the end of July and then flowered from August until October. In September, the first fruits were observed which matured between October and November. Galls formed in the plants during the months of October to November and remained during winter. The arrival of cold weather led to some leaf senescence in the plant (Figure 1).
A total of 2,249 individuals were collected, corresponding to 11 Orders of arthropods (Tables 1, 2). The most abundant family was Miridae (968 individuals), in which Macrolophus melanotoma (Costa, 1853) predominated, followed by the Aphididae family (364 individuals) represented by Brachycaudus sp. Hymenoptera parasitoids (222 individuals), composed of 15 families, were the third most abundant group, followed by the Formicidae family (167 individuals), with six genera (predominantly Plagiolepis sp.), the Order Araneae (149 individuals), with seven families, being (predominantly Oxyopidae and Thomisidae), and the family Aleyrodidae (142 individuals), mostly made up of Trialeurodes sp.
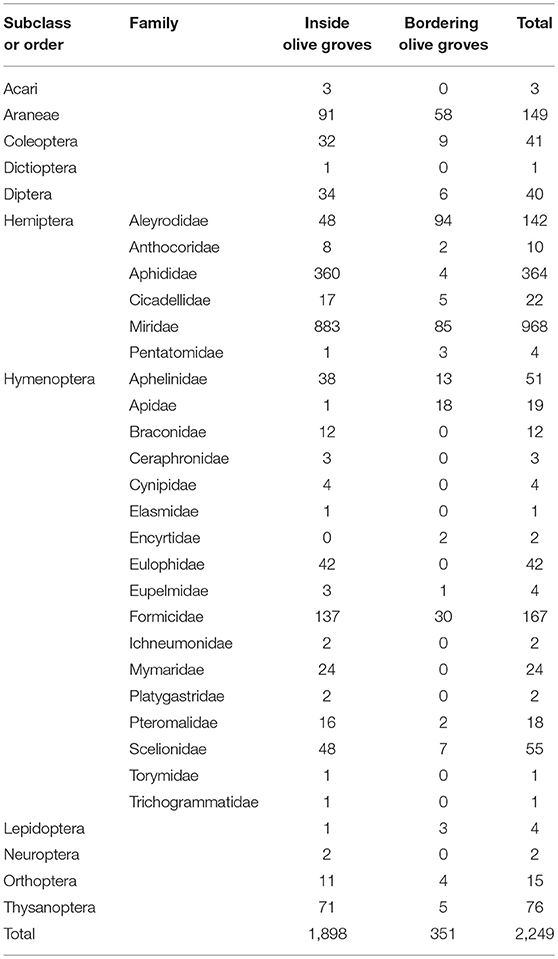
Table 1. Number of individuals collected during sampling period in bordering and inside olive grove.
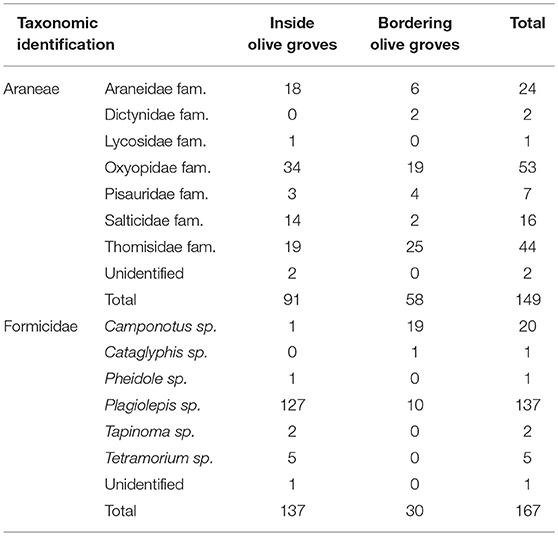
Table 2. Number of individuals collected to the Order Araneae and the family Formicidae during sampling period inside and bordering olive groves.
The post-flowering period was not analyzed due to the small number of captures, which accounted for 3.7% (83 individuals) of total arthropods collected.
We studied the most abundant groups of arthropods (Aphididae, Aleyrodidae, Araneae, Formicidae, Miridae and Hymenoptera parasitoids), which accounted for 89.5% (2,012 individuals) of total captures.
During the pre-flowering stage, in the most abundance taxonomic groups, a total of 196 arthropods were captured, while the number of arthropods captured dropped to 100 during the flowering stage.
The various groups of arthropods responded differently to the phenological changes in D. viscosa. The Aleyrodidae family occupied a predominant position during the pre-flowering period, although the presence of flowers led to a significant reduction in their populations (Kruskal-Wallis χ2 = 6.31; d.f. = 1; p < 0.05). The abundance of the other groups (Hymenoptera parasitoids, aphids, and predators) was not found to be affected by phenological changes in D. viscosa. Populations of the Miridae family remained high in the pre-flowering and flowering stages (Table 3).
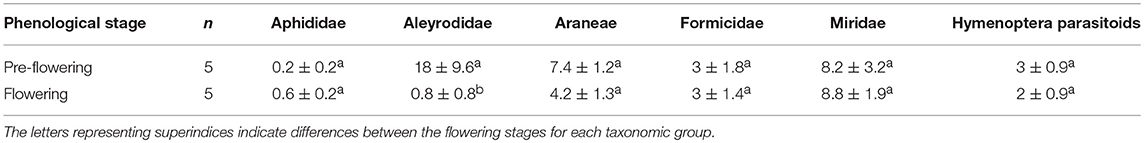
Table 3. Average number (mean ± SE) of individuals of different taxonomic groups captured during the pre-flowering and flowering stages of D. viscosa bordering olive groves.
In the pre-flowering stage, we observed a significantly negative correlation between the abundance levels of Araneae and Aphididae (Pearson cor = −0.94, p < 0.05), while the correlation between Aleyrodidae and Formicidae was positive (Pearson cor = 0.97, p < 0.01) (Table 4). During the flowering period, a significantly negative correlation between abundances of spiders and parasitoids was observed (Pearson cor = −0.96, p < 0.05).
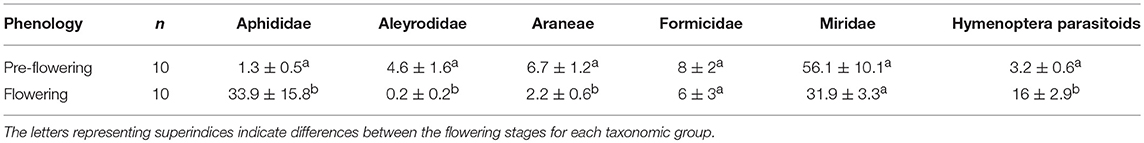
Table 4. Average number (mean ± SE) of individuals of different taxonomic groups captured before and during the flowering period of D. viscosa located inside the olive groves.
The number of individual arthropods captured in the most abundance taxonomic groups during the pre-flowering period reached 794, which increased to 904 individuals during the flowering period.
The populations of Aleyrodidae and Aphididae, the principal phytophage families present, were found to be affected in opposite ways by flowering. In the case of the Aleyrodidae family, as observed in D. viscosa plants bordering the olive groves, its populations diminished significantly (Kruskal-Wallis χ2 = 9.15; d.f. = 1; p < 0.01), while Aphididae populations were observed to rise considerably in this period (Kruskal-Wallis χ2 = 10.74; d.f. = 1; p < 0.01) (Table 4). Hymenoptera parasitoid populations, which multiplied 5-fold, were significantly higher during the flowering period (Kruskal-Wallis χ2 = 13.25; d.f. = 1; p < 0.001). As for the response of the different predator groups, the abundance of spiders was observed to diminish significantly in this period (Kruskal-Wallis χ2 = 8.81; d.f. = 1; p < 0.01); populations of the Formicidae family were similar in both periods, while those of the Miridae family, which reached high levels in both periods, declined, with no significant inter-period differences being observed (Table 4).
In the pre-flowering period, we noted a significantly positive correlation between Aphididae and Miridae (Pearson cor = 0.86, p < 0.01). During the flowering stage, a positive correlation was observed between Formicidae and Hymenoptera parasitoids (Pearson cor = 0.73, p < 0.05).
With respect to the comparison between cultivated and naturally growing D. viscosa plants, we observed that, among the majority taxonomic groups, during the pre-flowering and flowering periods in the cultivated plants, 354 and 353 individuals were captured, respectively; on the other hand, in the naturally growing plants, the numbers rose to 440 and 551 individuals, respectively. During the pre-flowering period, the abundances of the Aleyrodidae and Aphididae families were low, although Aleyrodidae populations were significantly higher in the cultivated plants (Fisher-LSD MSerror = 16.55; p < 0.05), while Aphididae populations were higher in the natural plants (Kruskal-Wallis χ2 = 6.91; d.f. = 1; p < 0.01). Populations of the other groups were similar in both types of plants (Figure 2). In addition, during the pre-flowering stage of natural D. viscosa plants, a positive correlation was observed between Aphididae and Miridae (Pearson cor = 0.91, p < 0.05), while no significant correlation in cultivated plants was found.
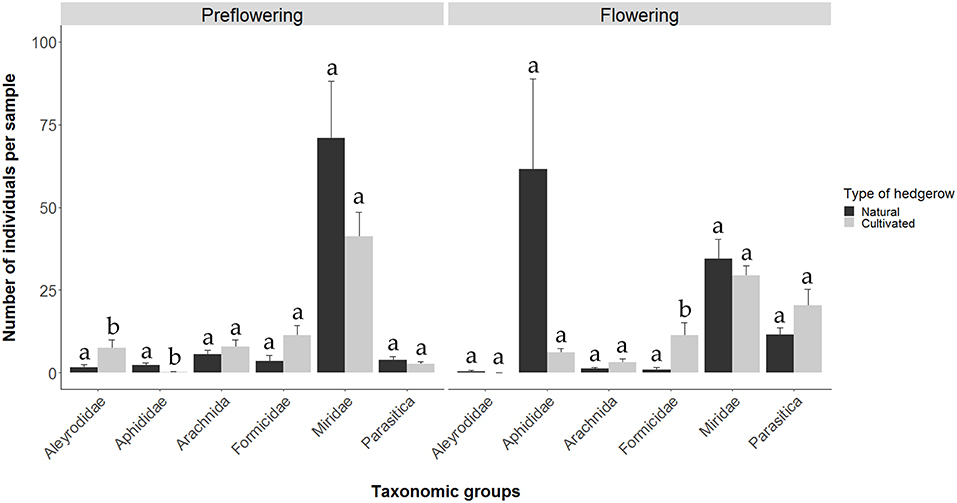
Figure 2. Number of individuals (media ± SE) of the different taxonomic groups during the pre-flowering and flowering periods of D. viscosa plants in natural and cultivated hedgerows inside olive groves.
During the flowering period, the abundance of the Formicidae family in cultivated plants was higher than that in natural plants (Kruskal-Wallis χ2 = 6.99; d.f. = 1; p < 0.01). The other groups analyzed (Aphididae, Aleyrodidae, Araneae, Miridae and Hymenoptera parasitoids) did not show any significant differences between the two types of plants (Figure 2). With respect to relationships between the groups in the natural plants, a negative correlation between aphids and Hymenoptera parasitoids was observed (Pearson cor = −0.89, p < 0.05). In cultivated plants, we found positive correlations between Aleyrodidae and Araneae (Pearson cor = 41, p < 0.05), between Araneae and Formicidae (Pearson cor = 0.64, p < 0.001) and Miridae (Pearson cor = 0.6, p < 0.001) and between Miridae and Hymenoptera parasitoids (Pearson cor = 0.47, p < 0.01).
D. viscosa plants adapted well to conditions in the areas studied, and their phenology did not differ significantly from other Mediterranean regions (Parolin et al., 2013). However, their phenology can be affected by agricultural vegetation cover management, as the timing and frequency of clearance can affect the plant's growth and floral development. Thus, late vegetation cover management in summer reduces the quantity of flowers (Simões et al., 2013) and prevents leaf senescence in winter (Parolin et al., 2013).
Throughout its life cycle, D. viscosa is colonized by numerous arthropods from different functional groups, whose populations can vary depending on requirements. The leaves, present from the beginning of April to December, can be used as food, whose importance for arthropod colonizers has not been analyzed (Parolin et al., 2014). The pollen and nectar of its open flowers are important sources of food for beneficial arthropods, as the nectar contains high concentrations of sugars (Hidalgo and Cabezudo, 1995), which, together with the scarcity of other flowering plants at that time of year in different agroecosystems, make D. viscosa a plant of considerable importance (Nave et al., 2017). Due to gall production by M. stylatus, the numerous larvae inside the flower during winter can be used as hosts by different families of Hymenoptera parasitoids, whose adults begin to emerge during the month of May (Franco-Micán et al., 2010; Mota et al., 2011).
Aleyrodidae are an abundant family in the group of phytophages associated with D. viscosa (Parolin et al., 2013; Rodríguez et al., 2018). In our study, Trialeurodes sp. was found to be present in both olive groves studied, preferentially during the pre-flowering period. This is in line with the finding of Rodríguez et al. (2018), who reported that no whiteflies were captured during the flowering period, which explains why Trialeurodes sp. may mostly feed on leaves during the pre-flowering period. Its positive correlation with the Formicidae family could be due to honeydew secreted by this aleyrodid. Another noteworthy phytophage is the aphid, belonging to the genus Brachycaudus sp., whose populations increased during the flowering period of D. viscosa plants located inside the olive groves. In the pre-flowering stage, we observed a positive correlation with the Miridae family, one of the principal enemies of the family Aphididae (Perdikis et al., 2007). Although an extract of D. viscosa showed aphid antifeedant activity (Mamoci et al., 2012), this plants species was colonized by various aphid species. These aphids can be parasitized by individuals belonging to the Aphelinidae family, as D. viscosa is considered a reservoir of aphid parasitoids (Kavallieratos et al., 2002).
The group of Hymenoptera parasitoids associated with D. viscosa in our study is highly complex, with 15 families, notably Scelionidae, Aphelinidae, and Eulophidae, having been identified. Their presence during both the pre-flowering and flowering stages of plants bordering and inside the olive groves points to their potential role in maintaining these natural enemies together with others associated with D. viscosa galls in different locations (Alcalá Herrera et al., 2017). In plants located inside olive groves, Hymenoptera parasitoid populations increased significantly during the flowering period. This could be due to the ability of adult Hymenoptera parasitoids to feed on pollen and nectar as well as to find possible hosts; although D. viscosa flowers have protected nectaries, the size of their corolla (5.91 mm deep and 1.19 mm wide) permits parasitoids, such as Ageniaspis fuscicollis (Hymenoptera: Encyrtidae) and Elasmus flabellatus (Hymenoptera: Eulophidae), to enter the corolla in order to feed (Nave et al., 2016).
Predators belonging to the Miridae family, principally M. melanotoma, were highly abundant in D. viscosa plants both bordering and inside olive groves; however, in other studies, mirid bugs were found to be more abundant in outer rows with higher whitefly densities (Alomar et al., 2002). M. melanotoma is present during pre-flowering and flowering periods. In Greece, Macrolophus sp. is present throughout the year in D. viscosa, reaching maximum levels in June and July, as it can feed on the plant and different types of prey (Perdikis et al., 2007). The small number of mirids found in the post-flowering stage were mirid nymphs, which is in line with the situation observed in the north of Spain, where winter populations of M. melanotoma are mostly composed of nymphs (Alomar et al., 1994). In pre-flowering plants situated inside the olive groves, mirid populations correlated with the Aphididae family, which constitutes the best prey for the development of mirids, while predator numbers were found to respond to the presence of aphids, such as Capitophorus inulae in spring (Perdikis et al., 2007). Another group of predators, spiders, was present in the areas studied, particularly during the pre-flowering stage in plants located inside the olive groves, possibly because they usually respond to fluctuations in their prey, as availability of food is a critical factor in their relationships (Picchi et al., 2016). The spiders present in olive groves are highly diverse, and, although most are generalist, some species specialize in certain prey (Cárdenas et al., 2011). Ants, whose populations vary throughout the year and are affected by vegetation cover management, are known to constitute a highly abundant group in olive groves (Redolfi et al., 1999). In our study, ant populations captured in D. viscosa did not differ significantly between the pre-flowering and flowering stages in either location, possibly due to their capacity to feed on many different types of food (Way and Khoo, 1992).
Differences between cultivated and natural plants were mainly observed in the pre-flowering stage, which affected both phytophage groups, with the Aphididae family showing the highest abundance in natural plants and the Aleyrodidae family in cultivated plants. This could be due to differences in existing plant diversity surrounding D. viscosa plants.
Our findings clearly show that arthropod communities associated with D. viscosa and the relationships between different groups of arthropods can vary during the pre-flowering and flowering stages. However, in all locations studied (inside and bordering olive groves) and in cultivated and natural plants, D. viscosa is still potentially of considerable interest as a reservoir of different predators and Hymenoptera parasitoids, although it is important to point out that none of the phytophages present affected the olive trees. Nevertheless, it should be noted that, in olive groves and commercial plantations, D. viscosa, which is very difficult to remove using herbicides and has significant resprouting capacity, is considered to be a problematic plant (Simões et al., 2013); these two factors should therefore be taken into account when D. viscosa is incorporated into the ecological infrastructure.
All relevant data generated and anlyzed for this study are included in the manuscript.
MC obtained the funding. MC and JC-R conceived and designed the study. MC, MF-S carried out the sampling. RA and MF-S identified the arthropod. RA carried out the formal analyses. MC, JC-R, and RA wrote, reviewed, and edited the manuscript. The manuscript was revised and approved by all the authors.
This study was funded within the framework of a Spanish National Research Council (CSIC) intramural funding program (Project 201540E007) and by the Junta de Andalucía (Project P12-AGR-1419). The Spanish Council for Scientific Research CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI) assisted with the payment of the publication fee.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thank Nuria Agustí who identified the Macrolophus specimen using molecular techniques. Joaquin Moreno Chocano for his help to sampling in field. The manuscript was translated by Michael O'Shea.
Alcalá Herrera, R., Castro Rodríguez, J., Fernández-Sierra, M. L., Moreno-Chocano, J., and Campos Aranda, M. (2017). “Incidencia de la localización de Dittrichia viscosa (Asteraceae) en el olivar sobre el complejo parasitario asociados a las agallas producidas por Myopites stylata (Diptera: Tephritidae),” in XVIII Symposium Científico-Técnico Expoliva del 10 al 12 de mayo de 2017 (Jaén: Fundación Olivar, 1–3.
Alcántara, C., Soriano, A., Saavedra, M., and Gómez, J. A. (2017). “Sistemas de manejo del suelo,” in El cultivo del olivo, 7th Edn., eds D. Barranco Navero, R. Fernández Escobar, and L. Rallo Romero (Madrid: Mundi-Prensa, 335–417.
Alomar, O., Goula, M., and Albajes, R. (1994). Mirid bugs for biological control: identification, survey in non-cultivated winter plants, and colonization of tomato fields. IOBC/WPRS Bull. 17, 217–223.
Alomar, O., Goula, M., and Albajes, R. (2002). Colonisation of tomato fields by predatory mirid bugs (Hemiptera: Heteroptera) in northern Spain. Agric. Ecosyst. Environ. 89, 105–115. doi: 10.1016/S0167-8809(01)00322-X
Araj, S. E., and Wratten, S. D. (2015). Comparing existing weeds and commonly used insectary plants as floral resources for a parasitoid. Biol. Control 81, 15–20. doi: 10.1016/j.biocontrol.2014.11.003
Barrientos, J. A. (1988). Bases para un curso práctico de entomología. Barcelona: Asociación Española de Entomología.
Bianchi, F. J., Booij, C. J., and Tscharntke, T. (2006). Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci. 273, 1715–1727. doi: 10.1098/rspb.2006.3530
Cárdenas, M., Campos, M., and Pascual, F. (2011). Roles de las arañas (Orden Araneae) en el agroecosistema del olivar. Phytoma España 229, 1–6.
Carrié, R. J., George, D. R., and Wäckers, F. L. (2012). Selection of floral resources to optimise conservation of agriculturally-functional insect groups. J. Insect Conserv. 16, 635–640. doi: 10.1007/s10841-012-9508-x
Castañé, C., Agustí, N., Arnó, J., Gabarra, R., Riudavets, J., Comas, J., et al. (2013). Taxonomic identification of Macrolophus pygmaeus and Macrolophus melanotoma based on morphometry and molecular markers. Bull. Entomol. Res. 103, 204–215. doi: 10.1017/S0007485312000545
Chaplin-Kramer, R., De Valpine, P., Mills, N. J., and Kremen, C. (2013). Detecting pest control services across spatial and temporal scales. Agric. Ecosyst. Environ. 181, 206–212. doi: 10.1016/j.agee.2013.10.007
De Mendiburu, F. (2017). Agricolae: Statistical Procedures for Agricultural Research. R package version 1.2-8 Edn.
Franco-Micán, S. X., Castro, J., and Campos, M. (2010). Preleminary study of the parasitic complex associated with Dittrichia viscosa in Andalusia (Spain). Integrated Protection of Olive Crops. IOBC/WRPS Bulletin 53, 139–143.
Gómez, J. A., Campos, M., Guzman, G., Castillo-Llanque, F., Vanwalleghem, T., Lora, A., et al. (2018). Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ. Sci. Pollut. Res. 25, 977–989. doi: 10.1007/s11356-016-8339-9
Goulet, H., and Huber, J. T. (1993). Hymenoptera of the World: An Identification Guide to Families. Ottawa, ON: Centre for Land and Biological Resources Research.
Hidalgo, M. I., and Cabezudo, B. (1995). Producción de néctar en matorrales del sur de España (Andalucía). Acta Botanica Malacitana 20, 123–132.
Holland, J. M., Bianchi, F. J. J. A., Entling, M. H., Moonen, A. C., Smith, B. M., and Jeanneret, P. (2016). Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag. Sci. 72, 1638–1651. doi: 10.1002/ps.4318
Holland, J. M., Douma, J. C., Crowley, L., James, L., Kor, L., Stevenson, D. R. W., et al. (2017). Semi-natural habitats support biological control, pollination and soil conservation in Europe. A review. Agron. Sustain. Dev. 37:31. doi: 10.1007/s13593-017-0434-x
Kavallieratos, N. G., Stathas, G. J., Athanassiou, C. G., and Papadoulis, G. T. (2002). Dittrichia viscosa and Rubus ulmifolius as reservoirs of aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) and the role of certain coccinellid species. Phytoparasitica 30, 231–242. doi: 10.1007/BF03039992
Lambion, J. (2011). “Functional biodiversity in Southern France: a method to enhance predatory mirid bug populations,” in I International Conference on Organic Greenhouse Horticulture. Acta Horticulturae 915, eds M. Dorais and S. D. Bishop (Bleiswijk: International Society for Horticultural Science), 165–170. doi: 10.17660/ActaHortic.2011.915.20
Landis, D. A., Wratten, S. D., and Gurr, G. M. (2000). Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann. Rev. Entomol. 45, 175–201. doi: 10.1146/annurev.ento.45.1.175
Lykouressis, D., Perdikis, D., and Kallioras, C. (2012). Selection of Macrolophus melanotoma between its main non-crop host plant (Dittrichia viscosa) and eggplant, pepper and tomato, in choice experiments. Entomol. Hellenica 21, 3–12. doi: 10.12681/eh.11513
Mamoci, E., Cavoski, I., Andres, M. F., Díaz, C. E., and Gonzalez-Coloma, A. (2012). Chemical characterization of the aphid antifeedant extracts from Dittrichia viscosa and Ferula communis. Biochem. Syst. Ecol. 43, 101–107. doi: 10.1016/j.bse.2012.02.012
Mata, L., and Goula, M. (2011). Clave de Familias de Heterópteros de la Península Ibérica (Insecta, Hemiptera, Heteroptera). Versión 1. Barcelona, ES: Centre de Recursos de Biodiversitat Animal, Facultat de Biologia, Universitat de Barcelona.
Miñarro, M., and Prida, E. (2013). Hedgerows surrounding organic apple orchards in north-west Spain: potential to conserve beneficial insects. Agric. For. Entomol. 15, 382–390. doi: 10.1111/afe.12025
Mota, L., Bento, A., Porcel, M., Campos, M., and Pereira, J. A. (2011). “O papel de Dittrichia viscosa (L.) W. Greuter no olival: estudo da influencia dos parámetros biométricos das galhas no número Myopites stylatus (Fabricius) e seus parasitoides,” in VII Congreso Nacional de Entomología Aplicada (Baeza), de 24 al 28 de Octubre de 2011, 128.
Nave, A., Crespi, A. L., Goncalves, F., Campos, M., and Torres, L. (2017). Native Mediterranean plants as potential food sources for natural enemies of insect pests in olive groves. Ecol. Res. 32, 459–459. doi: 10.1007/s11284-017-1460-5
Nave, A., Gonçalves, F., Crespí, A. L., Campos, M., and Torres, L. (2016). Evaluation of native plant flower characteristics for conservation biological control of Prays oleae. Bull. Entomol. Res. 106, 249–257. doi: 10.1017/S0007485315001091
Orfanidou, C. G., Maliogka, V. I., and Katis, N. I. (2016). False Yellowhead (Dittrichia viscosa), a banker plant as source of tomato infectious chlorosis virus in Greece. Plant Dis. 100, 869–869. doi: 10.1094/PDIS-10-15-1201-PDN
Paredes, D., Cayuela, L., and Campos, M. (2017). “Potential of ecological infrastructures to restore conservation biological control: case study in Spanish olive groves,” in Natural Enemies. Identification Protection Strategies and Ecological Impacts, ed. S. A. P. Santos (New York, NY: Nova Publishers), 153.
Parolin, P., Scotta, M. I., and Bresch, C. (2014). Biology of Dittrichia viscosa, a Mediterranean ruderal plant: a review. Phyton-Int. J. Exp. Bot. 83, 251–262.
Parolin, P., Scotta, M. I., and Bresh, C. (2013). Notes on the phenology of Dittrichia viscosa. J. Mediterr. Ecol. 12, 27–35.
Perdikis, D., Favas, C., Lykouressis, D., and Fantinou, A. (2007). Ecological relationships between non-cultivated plants and insect predators in agroecosystems: the case of Dittrichia viscosa (Asteraceae) and Macrolophus melanotoma (Hemiptera: Miridae). Acta Oecol. Int. J. Ecol. 31, 299–306. doi: 10.1016/j.actao.2006.12.005
Picchi, M. S., Bocci, G., Petacchi, R., and Ending, M. H. (2016). Effects of local and landscape factors on spiders and olive fruit flies. Agric. Ecosyst. Environ. 222, 138–147. doi: 10.1016/j.agee.2016.01.045
Porcel, M., Cotes, B., Castro, J., and Campos, M. (2017). The effect of resident vegetation cover on abundance and diversity of green lacewings (Neuroptera: Chrysopidae) on olive trees. J. Pest Sci. 90, 195–196. doi: 10.1007/s10340-016-0748-5
R Development Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Redolfi, I., Tinaut, A., Pascual, F., and Campos, M. (1999). Qualitative aspects of myrmecocenosis (Hym., Formicidae) in olive orchards with different agricultural management in Spain. J. Appl. Entomol. Zeitschr. Angew. Entomol. 123, 621–627. doi: 10.1046/j.1439-0418.1999.00411.x
Rodríguez, E., González, M., Paredes, D., Campos, M., and Benítez, E. (2018). Selecting native perennial plants for ecological intensification in Mediterranean greenhouse horticulture. Bull. Entomol. Res. 108, 694–704. doi: 10.1017/S0007485317001237
Simões, P. M., Belo, A. D. F., and Souza, C. (2013). Effects of mowing regime on diversity of mediterranean roadside vegetation – implications for management. Pol. J. Ecol. 61, 241–255.
Simon, S., Bouvier, J. C., Debras, J. F., and Sauphanor, B. (2010). Biodiversity and pest management in orchard systems. A review. Agron. Sustain. Dev. 30, 139–152. doi: 10.1051/agro/2009013
Tixier, M. S., Kreiter, S., Auger, P., Sentenac, G., Salva, G., and Weber, M. (2000). Phytoseiid mites located in uncultivated areas surrounding vineyards in three French regions. Acarologia 41, 127–140.
Valle Tendero, F., Navarro Reyes, F. B., Jiménez Morales, M. N., Algarra Ávila, J. A., Arrojo Agudo, E., Asensi Marfil, A., et al. (2005). Datos botánicos aplicados a la gestión del medio natural andaluz I: Bioclimatología y Biogeografía. Sevilla: Consejería de Medio Ambiente, Junta de Andalucía.
Villa Serrano, A. M. (2016). Ecological infrastructures in sustainable olive growing: studies about Prays oleae (Bernard) and its natural enemies (Doctoral thesis), University of Lisboa, Lisbon, Portugal.
Warlop, F. (2006). Limitation of olive pest populations through the development of conservation biocontrol. Cah. Agric. 15, 449–455.
Way, M. J., and Khoo, K. C. (1992). Role of ants in pest management. Ann. Rev. Entomol. 37, 479–503. doi: 10.1146/annurev.en.37.010192.002403
Zhang, W., Ricketts, T. H., Kremen, C., Carney, K., and Swinton, S. M. (2007). Ecosystem services and dis-services to agriculture. Ecol. Econ. 64, 253–260. doi: 10.1016/j.ecolecon.2007.02.024
Keywords: ecological infrastructure, Olea europaea, Hymenoptera parasitoids, Miridae, Araneae, Formicidae, Aleyrodidae
Citation: Alcalá Herrera R, Castro-Rodríguez J, Fernández-Sierra ML and Campos M (2019) Dittrichia viscosa (Asterales: Asteraceae) as an Arthropod Reservoir in Olive Groves. Front. Sustain. Food Syst. 3:64. doi: 10.3389/fsufs.2019.00064
Received: 07 May 2019; Accepted: 29 July 2019;
Published: 06 September 2019.
Edited by:
José Manuel Martínez-Rivas, Instituto de la Grasa (IG), SpainReviewed by:
Agnieszka Barbara Najda, University of Life Sciences of Lublin, PolandCopyright © 2019 Alcalá Herrera, Castro-Rodríguez, Fernández-Sierra and Campos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafael Alcalá Herrera, cmFmYS5hbGNhbGFAZWV6LmNzaWMuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.