- 1Department of Biological and Agricultural Engineering, University of California, Davis, Davis, CA, United States
- 2Department of Agricultural Engineering, Mansoura University, Mansoura, Egypt
- 3Department of Land, Air, and Water Resources, University of California, Davis, Davis, CA, United States
This study investigated the effects of applying anaerobically digested food waste and dairy manure-derived biofertilizers to processing tomatoes. The biofertilizers were produced from a pilot scale system consisting of coarse solid separation and ultrafiltration (5,000 Da) with a capacity of approximately 3.8 m3·d−1. The coarse solids had particle size >53 μm and were not used for drip fertigation. The liquid concentrate and permeate from the system were both delivered to tomato plants through a subsurface drip fertigation system in a farm-scale cultivation experiment. The results showed that liquid digestate biofertilizers could be effectively delivered to the tomato plants given that steps to ensure suitable particle sizes were maintained prior to delivery. The ultrafiltered dairy manure digestate biofertilizer (DMP) had the highest yield of red tomatoes (7.13 ton·ha−1) followed by the concentrated food waste digestate biofertilizer (FWC) and mineral N fertilizer treatments with 6.26 and 5.98 ton·ha−1, respectively. The FWC biofertilizer produced tomatoes with significantly higher total and soluble solids contents compared to the synthetically fertilized tomatoes. Few significant differences between the treatments were observed among the pH, color, or size of the red tomatoes. These results indicate promise for the prospect of applying digestate biofertilizer products to tomatoes using the industry standard subsurface drip fertigation method. Additionally, digestate-derived biofertilizers may have potential to increase crop yields as well as certain quality characteristics of the harvested tomato fruit. No changes in soil quality were found among treatments but more study is required to understand long-term effects of biofertilizer applications with regards to soil quality and environmental risks.
Introduction
The need to mitigate rising global greenhouse gas emissions demands intensive development of renewable energy sources as well as the recovery of value from historically underutilized resources. The Food and Agriculture Organization of the United Nations estimated that 1.3 billion tons of edible food is wasted globally each year resulting in 250 km3 of corresponding wasted water and an addition of 3.3 billion tons of carbon dioxide (CO2e) emissions to the atmosphere (FAO, 2013). The organic fractions of agricultural and municipal solid wastes pose an additional concern if disposed of in landfills where anaerobic decomposition releases methane and other pollutant gases to the atmosphere (Psomopoulos et al., 2009). Many US states, including California, face environmental challenges associated with the management of food waste and dairy manure. Approximately a third of landfilled material in California is organic with about 50% of that (5.5 million tons) made up of food waste (CalRecycle, 2015). These landfilled organics currently comprise about 2% (8.8 million tons of CO2e) of the total greenhouse gas emissions of California (CARB, 2017).
Vast quantities of nutrients exist in organic wastes that can potentially be utilized as fertilizers or soil amendments to subsidize the tremendous demand for synthetic chemical fertilizers and to reduce the economic and environmental costs associated with fertilizer production and waste disposal. Global greenhouse gas emissions from fertilizer production were estimated at up to 575 million tons of CO2e in 2007, about 4% of total anthropogenic greenhouse gas emissions that year (Vermeulen et al., 2012). Meanwhile, nitrate leaching from fertilizer and manure applications to cropland accounts for 83% of nitrate loading to California groundwater supplies (Harter et al., 2012). In addition to groundwater pollution, dairy farms account for 4.8% of California's total greenhouse gas emissions (CARB, 2017).
Organic materials, such as food waste and manure, are common substrates for the anaerobic digestion process (AD) to produce renewable energy and nutrient rich effluents. Anaerobic digestion systems may have the capacity to reduce CH4 and N2O emissions from dairy operations by ~60 and 70%, respectively (Montes et al., 2013). If the effluent from AD (digestate) is used as fertilizer, more environmental benefits may be realized including emission avoidance from synthetic fertilizers (Montes et al., 2013). Digestate has been shown to have excellent fertilizer potential with several studies showing similar or higher yields obtained when fertilized with digestate compared to synthetic fertilizer or undigested animal manures or slurries (reviewed by Nkoa, 2014). Additionally, some studies have suggested that controlled management of digestate can suppress soil borne diseases (Cao et al., 2016), aid in the inactivation of certain weed seeds when used in combination with soil solarization (Fernández-Bayo et al., 2017), as well as possibly reduce nutrient leaching while maintaining crop productivity (Walsh et al., 2012).
While anaerobic digestion has been shown to effectively inactivate some potential pathogens such as Escherichia coli and Salmonella, others such as Listeria and spore-formers have been reported to be less affected by the anaerobic digestion process (Goberna et al., 2011; Insam et al., 2015). Moreover, while digestate may offer excellent fertilizer potential due to its higher proportion of mineralized nutrients compared to undigested materials, attention must be paid to avoid over-applying digestate to land, which can cause negative environmental effects such as nutrient runoff or leaching, phytotoxicity, increased soil salinity, pathogen exposure, accumulation of heavy metals, and/or increased gaseous NH3 emissions (Alburquerque et al., 2012; Nkoa, 2014; Insam et al., 2015). Digestate contains carbon in the form of volatile fatty acids and complex organics from undigested particles (Franke-Whittle et al., 2014). Digestates have better abilities to sequester carbon in soils than pre-digested organic waste due to the lower portion of easily degradable substances in digestate (Maucieri et al., 2017). However, the delivery method of digestate may influence carbon sequestration potential and greenhouse gas emissions. It has been clearly shown that subsurface injection of digestate is preferential to minimize gaseous ammonia emissions from soil (Nkoa, 2014) but deeper application is also associated with increased penetration depths of ammonium and nitrate, which may pose a threat to groundwater quality (Lili et al., 2016). Even less is known about nitrous oxide and methane emissions from digestate application to soil (Möller, 2015).
The costs or profits that may be associated with digestate disposal or sale is highly variable and dependent on a variety of factors such as location (Delzeit and Kellner, 2013), targeted markets (Dahlin et al., 2015), and public and/or grower's perception of digestate (Dahlin et al., 2017). Processing of digestate to reduce volume, improve transportation economics, and recover nutrients in a more concentrated form may increase the feasible distances that digestate can be transported and help to prevent over-application of digestate in areas immediately surrounding digester plants (Fuchs and Drosg, 2013; Zarebska et al., 2014). Additionally, after large particles are removed, digestate can be applied through drip fertigation systems, which are used in 37% of all irrigated acreage in California and >60% of processing tomato acreage in California (Tindula et al., 2013; USDA FRIS, 2013). In addition to certain benefits of subsurface drip irrigation systems such as increased water use efficiency and the easier application of fertilizers (Camp, 1998), subsurface fertigation of digestate may also be useful in decreasing ammonia volatilization from fields following digestate application (Sommer and Hutchings, 2001; Lili et al., 2016). However, investigations relating to the performance of processed digestate as the main source of nutrients in subsurface drip fertigated cropping systems remains an under-studied area.
The worldwide tomato production reached 182 million tons in 2017 with regional production dominated by Asia (44.7%) and followed by Europe (23.3%) and the Americas (20.3%) (FAO, 2018). In 2015, tomatoes were California's seventh largest commodity market with $1.7 billion in sales, about 121,400 hectares of planted area throughout the state, and a total production of 13 million tons (CDFA, 2016; USDA, 2017). Tomato irrigation and fertilization is usually accomplished through subsurface drip lines with small emitters (e.g., 105 μm) that delivery water and nutrients directly to the plant roots (Hartz and Hanson, 2009). The two most important nutrients for tomato crops are nitrogen and potassium with average total crop uptakes of 280 kg N, 45 kg P, and 335–450 kg of K per hectare observed throughout a season (Hartz and Hanson, 2009). Due to the high levels of N and K normally found in digestates, it is likely that digestate could serve as a suitable fertilizer source for tomato. In a review of the literature, a few studies have investigated the use of digestate as a fertilizer for tomato in greenhouse (Qi et al., 2005; Vaughn et al., 2015; Stoknes et al., 2016) and field (Yu et al., 2010) experiments. The results of Yu et al. (2010) showed that digestate application increased the organic matter, available nutrients, and culturable microbes in the soil as well as contents of protein, soluble sugar, Vitamin C, and β-carotene in the tomato fruits. This experiment was performed in the field but included relatively small plot sizes (6 m × 1.8 m) and was managed with furrow irrigation, which is capable of accommodating materials with larger particles than drip fertigation, which faces challenges from emitter clogging in the presence of particles greater than the emitter opening size. Therefore, the feasibility of large-scale digestate biofertilizer application to tomato crops through drip irrigation remains uninvestigated.
Biofertilizers have been applied through drip fertigation before but most experiments consist of adding a supplement of a liquid formulation of microorganisms of interest, such as N fixers or P solubilizers (Abdelhamid et al., 2011). Although interest has recently been observed in the marketing of liquid biofertilizers suitable for drip-fertigation from a few companies in the US, there have been extremely few peer-reviewed investigations into the effects of these products on plant and soil health. Two studies have investigated the application of diluted poultry manure (Pibars et al., 2015) and swine lagoon wastewater (Ponce et al., 2002) through drip lines. Pibars et al. (2015) focused on investigating emitter clogging in response to the manure delivery but made no effort to reduce the clogging through filtration. Ponce et al. (2002) applied swine manure to tomato plants in soilless culture but little discussion was presented about the specifics of the drip system such as emitter size or filtration system. The soilless system used was also not representative of the industry standard for production of processing tomatoes.
In this study, we report the development and use of a digestate processing system that utilized solid-liquid separation and membrane filtration, with a capacity of 3.8 m3·d−1, for producing digestate-derived biofertilizer products. The objectives of this study were to (1) produce liquid biofertilizer products (concentrated and ultrafiltered) on the pilot scale from the digestate of a mesophilic dairy manure digester and a thermophilic food waste digester for crop production at the farm-scale, (2) examine the feasibility of applying digestate-derived liquid biofertilizer products through subsurface drip fertigation for the production of processing tomatoes, and (3) investigate the impact of biofertilizers on processing tomato yield and quality. It was hypothesized that (1) the digestate biofertilizer products would be suitable for delivery to tomatoes through subsurface drip lines; (2) the biofertilizer products could be used as the main fertilizer source for tomato plants and produce similar yields to mineral fertilizer controls; and (3) the biofertilizers could improve tomato quality. To our knowledge, the present study is the first of its kind to investigate the successful delivery of digestate biofertilizers through subsurface drip lines and to quantify, in a field scale trial, the effects of drip-fertigation of digestate biofertilizers on tomato crop yield and quality.
Materials and Methods
Digestate Collection and Processing
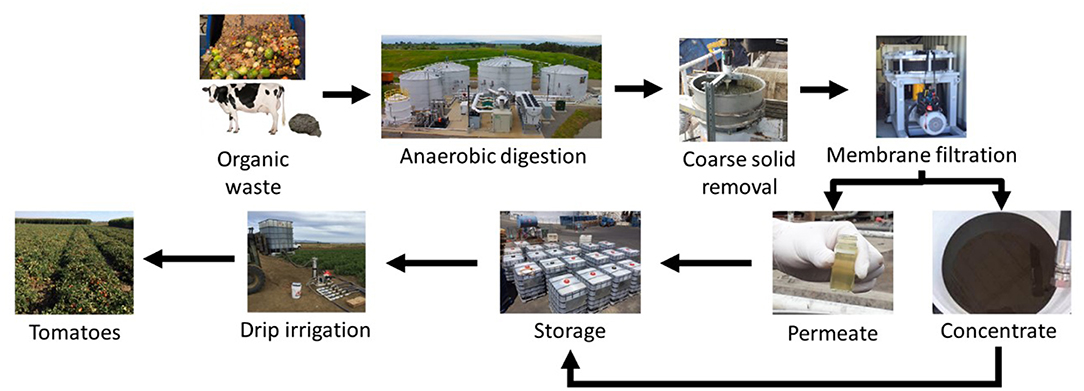
Digestate was collected from two separate facilities: (1) a mesophilic digester treating scraped dairy manure, and (2) a thermophilic digester treating mixed food waste. The digestates were stored in 26,000 L tanks and processed through a pilot separation and filtration system consisting of a SwecoTM vibratory screen (1 mm), a 53 μm self-cleaning screen, and a FMX antifouling ultrafiltration system (BKT Co. Ltd. Anaheim, CA). The general processing scheme can be seen in Figure 1. The filtration system was equipped with a 5,000 Da polyethersulfone membrane that incorporated nine sequential stacks of membranes with vortex-inducing rotating blades to reduce fouling. The unit consisted of three main lines: feed, permeate, and concentrate. The feed line was connected to a full tank of material, the permeate line was connected to a separate tote, and the concentrate (recycle) line was connected to the top of the original tank to allow for concentration of the digestates. When switching between digestate materials, the membrane system was flushed with at least 190 L of water and cleaned with 190 L of warm 1% Sodium hydroxide solution to prevent cross contamination between materials. The system was capable of processing about 3.8 m3·d−1 of raw digestate. Each produced material was analyzed for Total Kjeldahl Nitrogen (TKN), ammoniacal nitrogen (N-N), phosphorus, potassium, sodium, calcium, and magnesium by Denele Analytical Laboratories (Woodland, CA). The material at each step in the process was analyzed for Total Solids (TS) and volatile solids (VS) in the Bioenvironmental Engineering Research Laboratory at University of California, Davis (UC Davis) using standard methods (APHA et al., 2017). The permeate from the system was collected in totes and stored as liquid biofertilizer while the concentrate stream was allowed to continue until the desired concentration factor was achieved. In the end, the permeate and concentrate were 90 and 10% of the original volume, respectively. Both permeate and concentrate from the processing system were applied as liquid biofertilizer products to processing tomato plots (Figure 1).
The liquid biofertilizer storage totes were transported from the processing system to the test plots at the Russell Ranch Sustainable Agriculture Facility at UC Davis for application through drip lines. The liquid products were applied by fertigation using a high-pressure injection pump directly from the 1,250 L totes in which they were stored (Figures 2A,B). The liquid in the totes was hydraulically mixed prior to delivery to the field. The volume of digestate delivered was determined by an inline flow meter during delivery to the drip tape.

Figure 2. (A) Biofertilizer delivery including the tote storing biofertilizer material, a bag filter to ensure no particles larger than the emitters passed though, and a pump-driven injection system; (B) The tomato field before harvest; and (C) Solids collected from the 150 μm bag filter.
The drip tape was equipped with 105 μm emitters and 300 μm filters were placed upstream of the drip tape to filter out large particles that might clog the emitters. The biofertilizer products that were permeate from the ultrafiltration system were applied easily to the field but initial trials with the concentrate products revealed that larger particles had formed during storage. This necessitated further processing to reduce the particle size. The problematic materials (FWC and DMC) were diluted 2x with water and re-screened on-site with a sequential bag filtration strategy prior to application (within 60 min of filtration). The bag filter unit was equipped with 250, 200, 150, and 100 μm filter sizes and each was used individually and cleaned manually when fouling prevented efficient pumping (Figure 2C). Representative samples of each tote were taken after hydraulic mixing and analyzed for TKN prior to application in order to accurately quantify the nitrogen application rate.
Experimental Design
The experimental design for the tomato cultivation was a completely randomized block design. The block was chosen by region of the field to control for any lateral inconsistencies in soil conditions, sunlight exposure, water delivery, or other environmental factors that could influence the results of the experiment. The soil type for all experimental plots was Rincon silty clay loam. Five treatments of liquid digestate products were tested in four replicate plots each, with each plot spanning 64 m and 97.5 m2 (Figure S1). The experimental area did not include the first and last 4.6 m of each plot in order to avoid any inconsistencies in growth conditions at the edges of the plot. The treatments were: (1) no fertilizer (Ø), (2) mineral N fertilizer (UAN32), (3) dairy manure digestate permeate (DMP), (4) dairy manure digestate concentrate (DMC), and (5) food waste digestate concentrate (FWC). The mineral N fertilizer, UAN32, is a Urea-Ammonium-Nitrate product with 32% N content.
The planting, harvesting and product application schedule as well as the total nitrogen and volume application rates for tomatoes are presented in Table 1. Prior to transplanting, the field received a treatment consisting of 27.6 kg N, 36.1 kg P, 17.5 kg K, and 1.73 kg Zinc Chelate per hectare. The tomato (Lycopersicon esculentum Mill.) was transplanted on April 28th, 2016. The tomatoes were irrigated with 0.25 ha-m over the course of the experiment. The liquid biofertilizer products were delivered in four applications. Delivery pressure during and after fertigation was monitored for increases that could suggest obstruction of tubes or emitters but none were observed. By the final application, nitrogen was delivered at a rate of 202 kg N·ha−1, except for the DMC product. Unfortunately, the DMC product was abandoned after the first application due to sustained difficulties resulting from rapid particle agglomeration. The fertilization rate of 200 kg N·ha−1 has previously been found to be sufficient for optimal growth of drip-irrigated processing tomatoes in California (Hartz and Bottoms, 2009). Prior to fertilizer application and at the end of the tomato growing period, soil samples were taken randomly from the top 30 cm of soil in all treatment plots. Five replicated samples from each plot were mixed together in polyethylene bags and stored at 4 °C prior to analysis. The soil pH, NO3-N, N-N, Olsen-P, B, Zn, Fe, Cu, Mn, and S content of the soil samples were analyzed by a commercial laboratory (Denele Analytical Laboratories, Woodland CA).
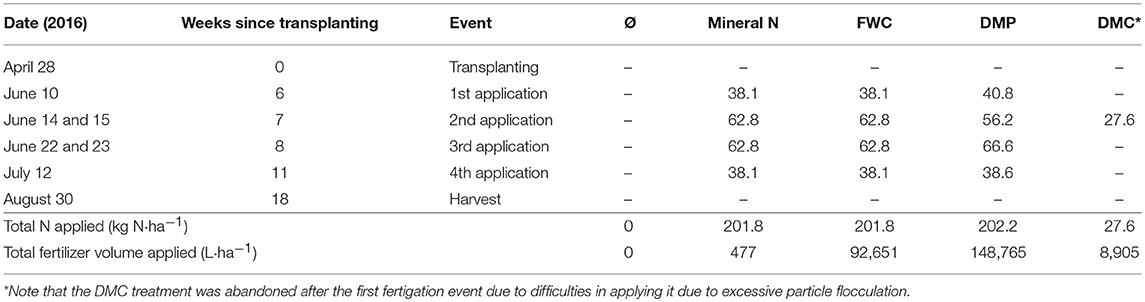
Table 1. Fertilizer application schedule of mineral N fertilizer (UAN-32), ultrafiltered dairy manure digestate (DMP), concentrated dairy manure digestate (DMC), and concentrated food waste digestate (FWC); amount of N delivered (kg N·ha−1); and volume of liquid fertilizers applied.
Tomato Harvest
Prior to harvest, tomato leaf chlorophyll content was measured with a portable SPAD meter. Thirteen measurements were taken from randomly selected leaves on both sides of each treatment plot at ~5 m intervals down the entire length of the plot. There were therefore 104 total leaf chlorophyll content measurements taken for each treatment category.
Tomatoes were harvested using a mechanical harvester. The mechanical harvester operated by cutting the whole plants at the ground and transporting the biomass up an inclined conveyor for processing to isolate the red tomatoes from the rest of the plant. The machine separated tomato fruit from non-fruit or vine biomass and vines were ejected from the machine back onto the field using a series of powerful vacuums. The fruit was sorted colorimetrically using an automated digital sensor. Green tomatoes were discarded by the machine while red tomatoes were collected into a bin for weighing.
During harvesting, representative samples of tomatoes before sorting were collected in 19 L buckets from each plot directly from the harvesting machine. The tomato samples were sorted into red, green, rotting, and sunburnt tomatoes. Each fraction was weighed and the percentage of each fraction was calculated. Additionally, about 30 red tomatoes from each plot were saved and stored at 4°C for further quality analysis.
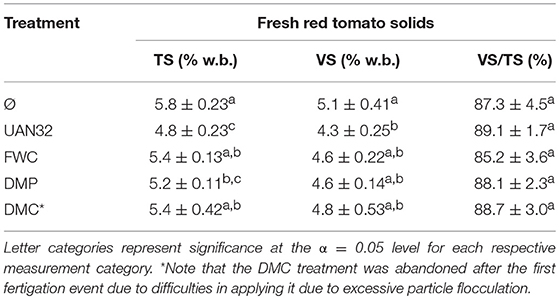
Fresh red tomato yield for each plot was measured onsite. The tractor-pulled collecting bin was tared before each trip down a plot and traveled beside the tomato harvester during harvesting, collecting red tomato biomass. The mass of the red tomatoes as well as plot length was recorded for each plot. For determination of dry red tomato yield, five of the tomatoes from each treatment plot were randomly sampled, cut, and pureed using a food processor. Total solids (TS) were determined by weighing 7–10 ml of puree into aluminum weigh dishes and drying overnight. Volatile solids (VS) were determined by burning the volatile fraction in a muffled furnace at 550°C for 3 h. Measurements were converted into ton·ha−1 yields by assuming a plot width of 1.5 m.
Red Tomato Quality Analysis
Red tomatoes were randomly chosen from those collected from each plot, washed, and wiped off using a paper towel. The tomatoes were then analyzed in lab for color, pH, soluble solids content (Brix), and size distribution. The color of tomato was determined in L*a*b* color space using Minolta Chroma Meter CR200 (Minolta Crop., Ramsey, NJ, USA). Tomato purees were centrifuged at 4,000·g for 5 min and the supernatant was filtered through a Whatman Grade 1 filter prior to soluble solids content measurement using a refractometer. The buckets taken from the field containing 125–155 tomatoes each were also subject to size classification. The tomatoes were manually sorted into 6 size groups using a PVC-plexiglass size distribution device with opening sizes of 39.4, 42.9, 45.5, 53.0, and 54.3 mm. After sorting, samples in each group were weighed and the number of tomatoes in each group was counted. The size distributions were determined in triplicate.
Statistical Analysis
All data were subjected to statistical analysis using R software. Statistically significant differences between treatments were analyzed using analysis of variance (ANOVA) and Tukey's range test at 5% significance level. Prior to ANOVA analysis, the Shapiro-Wilkes test was performed to ensure normality of all data and Levene's test was performed to ensure homogeneity of variance across samples. In the absence of normality or homogeneity of variance in the original data, Tukey's Ladder of Powers Transformation was performed to improve the data for ANOVA analysis (Tukey, 1977; Mangiafico, 2016).
Results
Chemical Properties of Liquid Digestate Biofertilizers
The nutrient and salt contents of the permeate and concentrate of the ultrafiltration unit used for processing the food waste and dairy manure digestates can be seen in Table 2. The concentrates had higher concentrations of most components with the exception of K and Na, which were similar or slightly lower in the concentrates compared to the permeates. The tendency of these monovalent ions to easily pass through to the permeate was also observed in lab-scale characterizations (Barzee et al., 2015). A higher portion of organic nitrogen was retained in the concentrate than the permeate with >90% of the nitrogen in the permeates being present as ammoniacal nitrogen. The concentrates were enriched in P, Ca, and Mg compared to the permeates, which was also expected from lab characterization studies (Barzee et al., 2015).
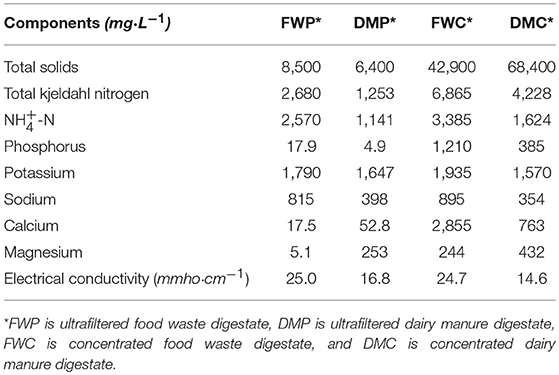
Table 2. Nutrient and salt concentrations of the ultrafiltered and concentrated digestate biofertilizer products.
Tomato Yield
The leaf chlorophyll content was estimated using SPAD measurements (Figure 3A). The average SPAD measurements ranged from 22.8 to 31.1 and large variation was observed between treatments. Differences in leaf SPAD measurements may be due to differences in growth stage, health, or nitrogen status of the tomato plants (Sandoval-Villa et al., 2002).
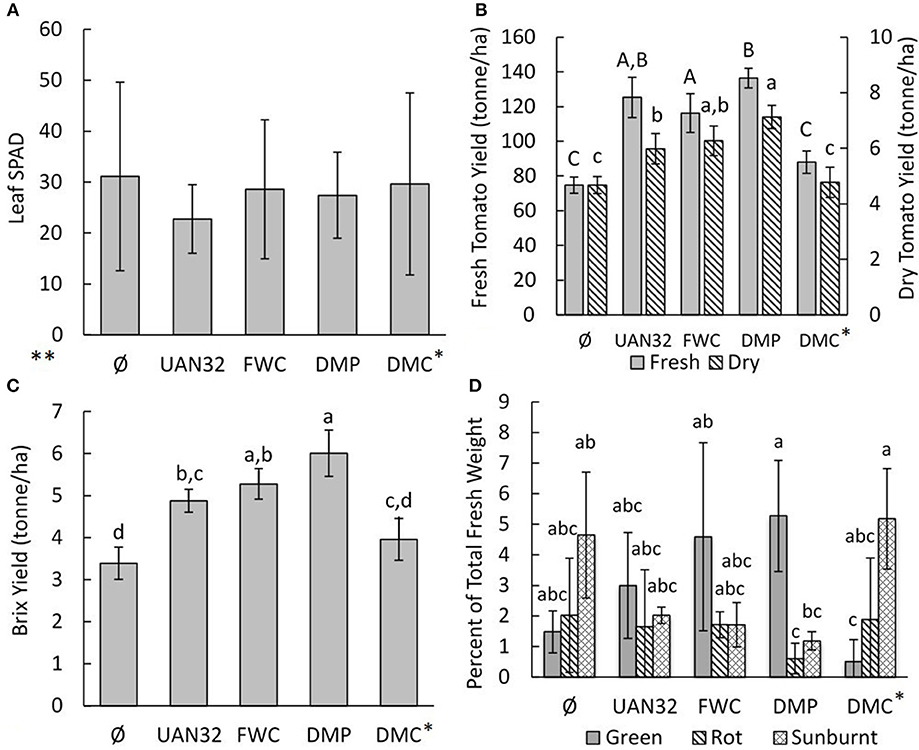
Figure 3. (A**) Chlorophyll SPAD measurements of the tomato plants treated with different fertilizers (n = 26, N = 104), (B) Fresh and dry red tomato yield (N = 4), (C) Brix yield of red tomato (N = 4), and (D) Percentage of total fruit obtained that had undesirable qualities (green, rot, sunburn) (N = 4). Letter categories represent significance at the α = 0.05 level. *Note that the DMC treatment was abandoned after the first fertigation event due to difficulties in applying it due to excessive particle flocculation. **Statistical analysis of leaf SPAD data is not presented as the data was highly non-normal and heteroscedastic.
The fresh red tomato yield was highest in the DMP treatment (136.4 tons·ha−1) followed by the mineral N fertilizer (125.3 tons·ha−1) and FWC (116.3 tons·ha−1) treatments (Figure 3B). The DMP treatment also had the maximum yield of dry red tomato biomass (7.13 ton·ha−1), which was significantly higher than the mineral N fertilizer positive control (5.98 ton·ha−1, p = 0.029). The dry yield of the concentrated food waste digestate (FWC) treatment was 6.26 ton·ha−1 and was statistically similar to both the DMP and positive control treatments even though its fresh yield was significantly lower than the DMP. The DMC treatment yield was low (4.77 ton·ha−1) and was due to the inadequate amount of fertilizer that was successfully applied (Table 1). The red tomato fruit total and volatile solids contents were highest in the unfertilized and biofertilizer treated plants and lowest in the mineral N fertilizer control (Table 3). The Brix yield results followed a similar trend to that of the dry tomato yield (Figure 3C) with the DMP treatment having the maximum yield (6.01 ton·ha−1), followed by FWC (5.28 ton·ha−1), and UAN32 (4.87 ton·ha−1). These values were generally in accordance with previous studies of tomatoes grown in similar regions (Hartz et al., 2005; Johnstone et al., 2005).
The distribution of fruits as red, green, rotten, and sunburnt was determined (Figure 3D). All treatments had 92–93% of all fruits made up of red tomatoes of sufficient quality for market with no significant differences (data not shown). Significant differences were observed in the percentage distribution of fruits in undesirable categories. The FWC and DMP treatments had significantly higher amounts of green tomatoes compared to the DMC treatment, which received little fertilizer (p < 0.03). The DMP treatment had significantly higher percentage of green tomatoes compared to rotten and sunburnt tomatoes (p < 0.03). The DMC treatment, on the other hand, had a significantly higher percentage of sunburnt fruit than green fruit (p = 0.0075) and a higher percentage of sunburnt fruit compared to the sunburnt or rotten fruit from the DMP treatment (p < 0.04). Generally speaking, more green tomatoes were observed on the fertilized plants and more sunburnt fruits were observed on unfertilized plants.
Tomato Quality
The quality of the red tomatoes was determined by measurements of pH, soluble solids (°Brix), and color (Table 4). The pH of tomato puree from all treatments was similar (one-way ANOVA p = 0.0788). The soluble solids in the filtered purees were also similar with the exception of the mineral N fertilizer treatment (UAN32) which had a slightly lower °Brix content. However, the relationship was only statistically significant for the comparison of the UAN32 and FWC treatments. The color of the red tomatoes collected were similar with few significant differences observed. The size distribution of red tomatoes was similar across all treatments with >90% of total tomato fruit by count and mass having a width <53 mm (Figure 4).
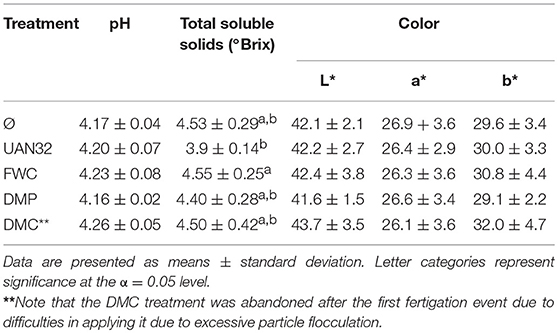
Table 4. Red tomato quality measurements of pH (N = 4), soluble solids (N = 4), and color (n = 10, N = 40).
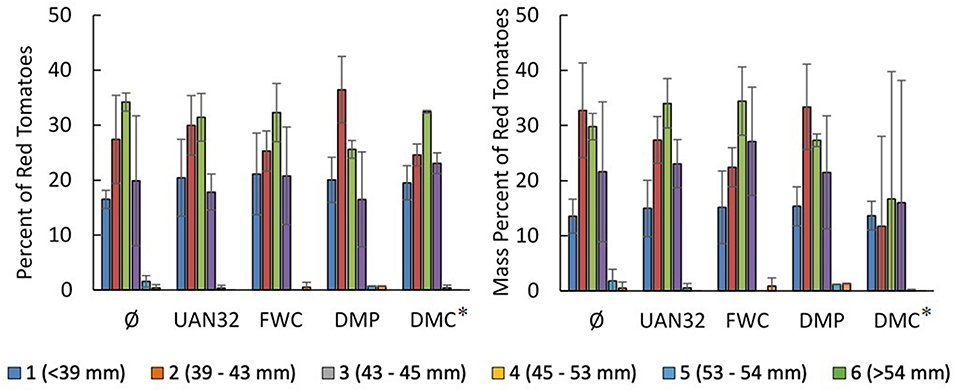
Figure 4. Size distribution of red tomatoes obtained as percentage of the total number of tomato fruits collected (Left) and the percentage of the total mass of tomato fruits (Right) (n = 3). *Note that the DMC treatment was abandoned after the first fertigation event due to difficulties in applying it due to excessive particle flocculation.
Soil Quality
The field soil was homogenized before the field trial and soil and chemical properties were consistent among treatments at the beginning of the season (Table S1). The average soil characteristics prior to fertilizer application were: pH of 6.7, EC of 1.4 mmho·cm−1, soluble salts of 914 ppm, NO3-N of 3.2 ppm, Olsen-P of 9.5 ppm, B of 0.2 ppm, Zn of 0.6 ppm, Fe of 8.1 ppm, Cu of 2.7 ppm, Mn of 8.7 ppm, and S of 99.9 ppm. The average soil characteristics at the end of the tomato season (Table 5) were: pH of 7.1, EC of 0.6 mmho·cm−1, soluble salts of 402 ppm, NO3-N of 4.1 ppm, Olsen-P of 8.1 ppm, B of 0.2 ppm, Zn of 0.3 ppm, Fe of 6.1 ppm, Cu of 1.3 ppm, Mn of 2.1 ppm, and S of 106 ppm.
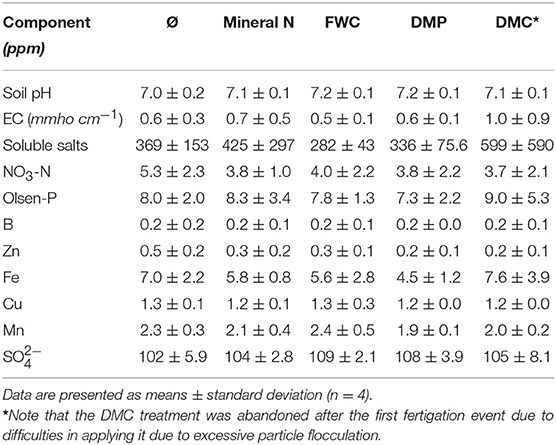
Table 5. Soil chemical properties in different strips of the tomato field experiment after fertilizer application and before harvest (August 22, 2016).
Discussion
Tomato Yield
Tomato plants that received full fertilization with the biofertilizers obtained similar or even higher fresh red tomato yields compared to the mineral N fertilizer treatment. Previous researches have indicated that the impacts of fertigated organic fertilizers on tomato yields were not consistent. Some researchers reported lower yields in organic fertilizer treatments compared to mineral N fertilizer treatments (Chassy et al., 2006; Pieper and Barrett, 2009; Yu et al., 2010; Bilalis et al., 2018) due to nitrogen loss to the environment (Cheng et al., 2004), relatively lower initial plant available nitrogen, and slow organic nitrogen mineralization (Hartz et al., 2010). On the other hand, some studies have also found similar or higher yields when digestate was used as a fertilizer (Chantigny et al., 2008; Möller et al., 2008; Nkoa, 2014; de Andrade Lima et al., 2018). The distinct yield differences could be due to the variation in mineral nitrogen contents of the organic amendments and different organic matter mineralization rates. Most studies evaluating yields of conventionally and organically grown tomatoes use compost as the organic fertilizer, which may have high proportions of organic nutrients that mineralize too slowly for the tomato demand (Bilalis et al., 2018). The method of fertilization can also play a large role in nutrient availability with some fertigation systems being capable of reducing nutrient loss (Rouphael et al., 2019). One study found a significantly lower (7–8%) yield in tomatoes that received livestock digestate in furrows compared to the synthetically fertilized control (Yu et al., 2010), possibly owing to accelerated ammonia volatilization of surface application of liquid organic fertilizer compared to subterranean application or with rapid incorporation to soil (Möller et al., 2008; Möller and Müller, 2012; Lili et al., 2016).
The dry red tomato yields for fully fertilized treatments were similar to previous researches in the same region (Hartz and Bottoms, 2009). Although the mean solids contents were higher for all treatments compared to the UAN32 treatment (Table 3), the FWC and DMP treatments could be due to increased production of organic and mineral matter, which was reported in a previous study of tomatoes grown on digestate (Yu et al., 2010) and is in agreement with another study that tested conventional vs. organic fertilization strategies with tomatoes (Pieper and Barrett, 2009). We speculate that the continuous application of high ionic strength biofertilizers can result in ion accumulation in soil (Munns, 2002; Rodríguez et al., 2019), which is associated with lower soil water potential. The lower soil water potential in biofertilizer treatments could have induced late-season water stress to the tomato plants and resulted in the higher soluble solids content (Johnstone et al., 2005). However, no significant differences were observed in the ratio of VS to TS in the red tomato fruit (Table 3), which may be expected to decrease if more salts were accumulated in the fruits. Another possible explanation for the higher solids production in the biofertilizer treatments may be from moderate salinity stress imposed from increased electrical conductivity of the soil from the digestate materials. Many studies have confirmed this physiological effect in tomatoes, which results in extended periods of starch accumulation, a reduction in root water absorption, and decreased fruit size (Moya et al., 2017; Rodríguez et al., 2019). However, no significant differences in fruit size were determined in this study. The increased percentage of premature (green) fruits in fertilized treatments (especially digestate fertilized treatments) may suggest that higher yields of red tomatoes may have been obtained with a longer cultivation period (Davis and Gardner, 1994). The increased distribution of sunburnt tomatoes in unfertilized or minimally fertilized treatments may be due to a decrease in canopy cover that exposed the tomatoes to sunlight more intensely.
Tomato Quality
Biofertilizers maintained or improved tomato quality parameters compared to the mineral N fertilizer control. The tomato fruit pH values observed in all treatments were similar to previous research (Tigist et al., 2013). The lower soluble solids concentration of the mineral N fertilizer treatment is in agreement with another study that found lower total and soluble solids contents in conventionally fertilized tomato plants and whose mechanism of effect may be similar to those described earlier for total solids contents (Pieper and Barrett, 2009). It should be noted, however, that differences in tomato age are known to influence °Brix content with plants of a higher distribution of younger fruits displaying higher soluble solids contents than older fruits (Renquist and Reid, 1998). This supports the notion that the digestate biofertilizer treatments had a higher percentage of younger fruit compared to the mineral N fertilizer control. Another study justified the increased °Brix content of organically-fertilized tomato plants as at least partially due to differences in physiological fruit maturity at time of harvest with the understanding that the actual mechanism of the increase may be dependent on many factors (Pieper and Barrett, 2009). Increased soluble solids concentrations observed in the unfertilized control and DMC treatments may be due to increased water evaporation from the fruits resulting from decreased canopy cover in these treatments (Davies and Hobson, 1981). The higher soluble solids contents observed in the FWC and DMP treatments were unlikely to be caused by evaporative losses since sunburnt fruits were not observed frequently (Figure 3D). Although a quantitative non-fruit biomass measurement was not undertaken in this study, visual inspection of the DMP, FWC, and synthetically fertilized plants revealed robust canopy cover while the canopies of the unfertilized and DMC plants were relatively less robust. For tomato size, while the type of fertilizer used may impact yield and other quality characteristics of the tomato plants, our data do not support the notion that changes in tomato size distribution are also connected to fertilizer type, which is in agreement with literature that suggests that size is mostly genetically determined (Pieper and Barrett, 2009; Bilalis et al., 2018). Interestingly, the absence of size differences among the digestate treatments contributes evidence against the notion that moderate salinity stress was the main contributor to the increased solids contents of these tomatoes since it is normally also associated with a decrease in fruit size (Moya et al., 2017).
Soil Quality and Agronomic Value
No significant differences were found in any of the soil quality measures among the treatments at the end of the growing season, including EC and soil pH (Table 5). These data do not support the suggestion that fertilization with digestate poses a greater risk of salt accumulation in soil than mineral fertilizer. However, due to the short time-scale of the study, long-term conclusions on this topic cannot be drawn. There is a disagreement in the literature about the effects of digestates on soil quality and it is likely that many possible impacts are linked to specific fertilization or irrigation systems. A 2 year greenhouse study that studied the application of pig slurry (EC between 7.5 and 21.1 mmho·cm−1) to calcareous soil growing green pepper, tomato, and lettuce found increased electrical conductivity and soluble salt concentrations in the 30 cm soil depth as a result of the slurry additions (Bernal et al., 1992). A field scale study growing tomatoes with digestate also found increased soil EC in the top 10–20 cm of soil from plots fertilized with digestate and mineral fertilizer (Yu et al., 2010). On the other hand, Odlare et al. (2008) conducted a 4 year field study using household-waste digestate to fertilize barley and oats and found negligible differences in soil physical properties and elemental contents (N, P, several metals) in digestate treatments compared to the controls. However, neither electrical conductivity nor soluble salt concentration were reported. Another field study in India using digestates to fertilize wheat crops found that digestate-amended soils had reduced bulk density and increased saturated hydraulic conductivity and moisture retention capacities (Garg et al., 2005). Long-term field trails with drip irrigation systems are necessary to understand the impact of repeated biofertilizer applications on soil chemical properties.
The agronomic value of digestate as a fertilizer and soil amendment has been a topic of study for years with the general scientific consensus in agreement of the potential of digestates as effective fertilizers and soil amendments (Nkoa, 2014). However, there is still progress to be made in understanding the unique characteristics of each digestate material and the methods for optimizing each material's nutrient use efficiency. The digestate chemical properties, as related to nutrient content and availability as well as salt content, depend on the anaerobic digester's feedstock and operation. The production of nutrient mineralization models for different digestate materials across different soil types and climates would allow for the targeted application of appropriate digestate fractions or the combination of digestate and mineral N fertilizers for an optimized delivery of nutrients that directly coincides with crop needs (Tambone and Adani, 2017). The uncertainty of N release dynamics of different digestate products in soil can create a challenge for the proper coupling of plant available N release and crop N demand. The concentrate and permeate products in this study could be utilized at different times of the season with the permeate providing immediately available nutrients with the concentrate providing a “slower-release” nutrient source with higher carbon content and soil amendment properties. If balanced properly, this strategy could provide sufficient nutrients for the crop while reducing nutrient leaching (Hartz and Bottoms, 2009). Many strategies and combinations of digestate materials are possible and the optimal combination for a given environmental scenario and agricultural practice will ultimately rely on a solid understanding of the material's agronomic properties and economics.
Challenges of Applying Digestate to Drip Lines
The delivery of digestate biofertilizers to crop fields poses a number of practical challenges for growers and fertilizer producers. There are clear benefits of subsurface delivery of fertilizers. Therefore, biofertilizers compatible with existing drip fertigation infrastructure will have high potential for high-value crops like processing tomatoes where drip fertigation is already a common practice. However, complex biofertilizers like digestate or manure contain biological material and nutrients that may support biofilm formation inside drip lines and also particles that must be removed prior to injection through the drip lines.
Although we timed the delivery of digestate biofertilizers to the drip lines to occur approximately halfway through an irrigation event to allow for large volumes of water to flush the lines, we didn't evaluate the long-term performance of the drip tape after repeated fertigation events. Extensive fouling of drip lines or emitters may prevent efficient delivery of nutrients and water to the crop roots and lead to decreased crop yields or, in extreme cases, crop losses. A main disadvantage of subsurface drip systems is the inability to directly observe water delivery from each emitter (Camp, 1998). These concerns may be amplified if biofertilizer application through drip lines increases the risk of emitter or line fouling, as has been suggested (Pibars et al., 2015). Therefore, the potential for biofilm formation and fouling of drip lines when using digestate biofertilizer materials must be comprehensively investigated and best practices for preventing the effects must be developed.
We observed rapid particle flocculation following filtration of digestate materials to 50 μm that complicated (in the case of FWC) or prevented (in the case of DMC) the successful delivery of the materials through the drip tape. The factors (e.g., ionic strength, particle size distribution, particle surface charge) contributing to the difference in the rate and/or extent of flocculation in the food waste and dairy manure digestate materials requires further investigation. Preventative measures to combat this effect need to be identified and quantified. Delivery of large particles in the FWC was avoided by injecting the material rapidly following filtration (within 1 h) but this strategy is not likely to be practical for commercial growers as it would necessitate on-site filtration equipment and additional labor costs. While it may be expected that the DMC product would perform similarly to the FWC product to produce excellent yields of high-quality tomatoes, it was not feasible to repeatedly filter the material quickly enough to allow delivery. Moreover, repeated filtration of digestate biofertilizers is undesirable as it removes potentially beneficial elements, such as organic nitrogen, phosphorus, magnesium, and calcium, that are commonly associated with small particle sizes (Barzee et al., 2015). Overall, more research is needed to optimize strategies for biofertilizer production, storage, and preparation for drip fertigation.
Economics and Commercialization
The economics of digestate processing for the production of biofertilizer products also deserves increased attention in order to understand the opportunities and barriers to commercialization. A wholistic economic analysis of the processing system used in this study is outside of the scope of the current work but the most significant capital and operational costs associated with this system are expected to be from the ultrafiltration system and transport of liquid products. A recent techno-economic analysis of several full scale digestate processing systems found specific capital and operational costs ranged between $6.06 and $7.83 per m3 of digestate processed (Bolzonella et al., 2017). The likely costs incurred for liquid hauling are measured by the distance fixed cost and distance variable cost (DFC and DVC, respectively). Studies of US and Canada-based markets have estimated the DFC and DVC costs (in 2018 US dollars) of liquid manure trucking to be 2.2–3.4 $·tonne−1 and 0.21–0.27 $·tonne−1km−1, respectively (Aillery et al., 2005; Ghafoori et al., 2007). While the permeate liquid fertilizer (DMP) tested in this study had the highest tomato yield and quality characteristics, probably owing to its highly mineralized nutrient content, it is also the most expensive material to transport due to its relatively dilute nutrient concentrations. More extensive processing, such as ammonia stripping or reverse osmosis filtration, could further concentrate this stream but would entail higher costs (Chiumenti et al., 2013; Bolzonella et al., 2017). The similar performance of the concentrated biofertilizer product to the permeate coupled with its higher nutrient concentrations suggests it as the more economical fertilizer for transport. However, it is unclear at this point what additional processing would be required to ensure this material's long-term suitability for drip fertigation systems. Studies on the economics of digestate processing technologies are available and highly useful but are mainly focused on European markets, where anaerobic digestion plants are more common than in the US (ADAS UK Ltd, 2013; Chiumenti et al., 2013; Delzeit and Kellner, 2013; Fuchs and Drosg, 2013; Drosg et al., 2015; Plana and Noche, 2016; Vaneeckhaute et al., 2016; Bolzonella et al., 2017). Research is needed to describe the economic conditions, costs, and availability of processing technology in the US case in order to understand the feasibility of increased US adoption of anaerobic digestion and digestate processing technologies as well as their associated biofertilizer products.
Conclusions
Digestate biofertilizers were effectively delivered to the tomato plants through a subsurface drip irrigation system given implementation of filtration steps to ensure suitable particle sizes are maintained prior to delivery. The red tomato yields from successful biofertilizer treatments were higher or similar compared to the synthetically fertilized controls. The concentrated food waste digestate biofertilizer produced tomatoes with significantly higher total and soluble solids contents compared to the synthetically fertilized tomatoes. The biofertilizer and mineral N fertilizers generally produced a larger proportion of green tomatoes while minimally fertilized treatments had greater proportions of sunburnt and rotten fruit. These results indicate promise for the prospect of applying digestate biofertilizer products to specialty crops like tomatoes using the industry standard subsurface drip fertigation method. Digestate-derived biofertilizers may have potential to increase crop yields as well as certain quality characteristics of the harvested tomato fruit. However, the processing and storage requirements of different digestate materials deserves more research in order to overcome challenges in biofertilizer application through drip fertigation systems. The long-term effects of digestate application on soil microbiota, nutrient and salt accumulation, and other soil quality measures is also needed to determine best practices for digestate application under different irrigation, soil, and cropping systems. Additional research should also be carried out to better understand the economics of different digestate processing options in the US, the agronomic properties and strategies for optimal nutrient use efficiency, and the environmental risks and benefits that might be associated with subsurface delivery of digestate biofertilizers through drip lines.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
TB, AE, HE-M, DW, KS, and RZ contributed conception and design of the study. TB, AE, and DW performed the field scale experiments. TB, AE, and HE-M performed the laboratory analyses. TB performed the statistical analysis. TB wrote the first draft of the manuscript. All authors wrote sections of the manuscript and contributed to manuscript revision, read, and approved the submitted version.
Funding
Primary support for this work was provided by the California Department of Food and Agriculture (Agreement 13-0557-SA). This work was also supported by the USDA National Institute of Food and Agriculture, Hatch Project CA-2122-H, and USDA National Institute of Food and Agriculture Grant #11925159. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We acknowledge support from the UC Office of the President's Multi-Campus Research Programs and Initiatives (MR-15-328473) through UC Water, the University of California Water Security and Sustainability Research Initiative.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. Israel Herrera and the staff at the Russell Ranch Sustainability Agriculture Facility for their valuable assistance with data collection and field work. We also acknowledge the contributions of Baptiste Truffort, Bibiana Molinos, Emma Torbert, Sam Hornstein, Josh Rapport, and Caleb Adams to the success of this project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2019.00058/full#supplementary-material
Abbreviations
Ø, No fertilizer (negative control); DMP, Dairy manure digestate permeate; DMC, Dairy manure digestate concentrate; FWP, Food waste digestate permeate; FWC, Food waste digestate concentrate; Mineral N, UAN32 synthetic fertilizer (positive control).
References
Abdelhamid, M. T., Selim, E. M., and El-Ghamry, A. M. (2011). Integrated effects of bio and mineral fertilizers and humic substances on growth, yield, and nutrient contents of fertigated cowpea (Vigna unguiculata L.) grown on sandy soils. J. Agron. 10, 34–39. doi: 10.3923/ja.2011.34.39
Aillery, M., Noel, G., and Breneman, V. (2005). Technical Documentation of the Regional Manure Management Model for the Chesapeake Bay Watershed. United States Department of Agriculture.
Alburquerque, J. A., de la Fuente, C., Ferrer-Costa, A., Carrasco, L., Cegarra, J., Abad, M., et al. (2012). Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 40, 181–189. doi: 10.1016/j.biombioe.2012.02.018
APHA AWWA, WEF. (2017). “S.2540B: total solids dried at 103-105 deg C,” in Standard Methods for the Examination of Water and Wastewater, 23rd Edn., eds E. W. Rice, R. B. Baird, and A. D. Eaton (Washington, DC: American Public Health Association), 132.
Barzee, T., Zhang, R., Edalati, A., Rapport, J., and El-Mashad, H. (2015). “Sustainable bio-fertilizer production from anaerobically digested organic wastes,” in 2015 ASABE Annual International Meeting (St. Joseph, MI: American Society of Agricultural and Biological Engineers).
Bernal, M. P., Roig, A., Madrid, R., and Navarro, A. F. (1992). Salinity risks on calcareous soils following pig slurry applications. Soil Use Manag. 8, 125–129. doi: 10.1111/j.1475-2743.1992.tb00907.x
Bilalis, D., Krokida, M., Roussis, I., Papastylianou, P., Travlos, I., Cheimona, N., et al. (2018). Effects of organic and inorganic fertilization on yield and quality of processing tomato (Lycopersicon esculentum Mill.). Folia Hortic. 30, 321–332. doi: 10.2478/fhort-2018-0027
Bolzonella, D., Fatone, F., Gottardo, M., and Frison, N. (2017). Nutrients recovery from anaerobic digestate of agro-waste: techno-economic assessment of full scale applications. J Environ. Manag. 216, 111–119. doi: 10.1016/j.jenvman.2017.08.026
Cao, Y., Wang, J., Wu, H., Yan, S., Guo, D., Wang, G., et al. (2016). Soil chemical and microbial responses to biogas slurry amendment and its effect on Fusarium wilt suppression. Appl. Soil Ecol. 107, 116–123. doi: 10.1016/j.apsoil.2016.05.010
Chantigny, M. H., Angers, D. A., Belanger, G., Rochette, P., Eriksen-Hamel, N., Bittman, S., et al. (2008). Yield and nutrient export of grain corn fertilized with raw and treated liquid swine manure. Agron. J. 100, 1303–1309. doi: 10.2134/agronj2007.0361
Chassy, A. W., Bui, L., Renaud, E. N. C., Van Horn, M., and Mitchell, A. E. (2006). Three-year comparison of the content of antioxidant microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell peppers. J. Agric. Food Chem. 54, 8244–8252. doi: 10.1021/jf060950p
Cheng, J., Shearin, T. E., Peet, M. M., and Willits, D. H. (2004). Utilization of treated swine wastewater for greenhouse tomato production. Water Sci. Technol. 50, 77–82. doi: 10.2166/wst.2004.0093
Chiumenti, A., da Borso, F., Teri, F., Chiumenti, R., and Piaia, B. (2013). Full-scale membrane filtration system for the treatment of digestate from a co-digestion plant. Appl. Eng. Agric. 29, 985–990. doi: 10.13031/aea.29.10117
Dahlin, J., Herbes, C., and Nelles, M. (2015). Biogas digestate marketing: qualitative insights into the supply side. Resour. Conserv. Recycl. 104, 152–161. doi: 10.1016/j.resconrec.2015.08.013
Dahlin, J., Nelles, M., and Herbes, C. (2017). Biogas digestate management: evaluating the attitudes and perceptions of German gardeners towards digestate-based soil amendments. Resour. Conserv. Recycl. 118, 27–38. doi: 10.1016/j.resconrec.2016.11.020
Davies, J. N., and Hobson, G. E. (1981). The constituents of tomato fruit - the influence of environment, nutrition, and genotype. CRC Crit. Rev. Food Sci. Nutr. 15, 205–280.
Davis, J. M., and Gardner, R. G. (1994). Harvest maturity affects fruit yield, size, and grade of fresh-market tomato cultivars. HortScience 29, 613–615.
de Andrade Lima, M., Charalampopoulos, D., and Chatzifragkou, A. (2018). Optimisation and modelling of supercritical CO 2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 133, 94–102. doi: 10.1016/j.supflu.2017.09.028
Delzeit, R., and Kellner, U. (2013). The impact of plant size and location on profitability of biogas plants in Germany under consideration of processing digestates. Biomass Bioenergy. 52, 43–53. doi: 10.1016/j.biombioe.2013.02.029
Drosg, B., Fuchs, W., Al Seadi, T., Madsen, M., and Linke, B. (2015). Nutrient Recovery by Biogas Digestate Processing. IEA Bioenergy.
FAO (2018). FAOSTAT. Available online at: http://www.fao.org/faostat/en/#data/QC
Fernández-Bayo, J. D., Achmon, Y., Harrold, D. R., McCurry, D. G., Hernandez, K., Dahlquist-Willard, R. M., et al. (2017). Assessment of two solid anaerobic digestate soil amendments for effects on soil quality and biosolarization efficacy. J. Agric. Food Chem. 65, 3434–3442. doi: 10.1021/acs.jafc.6b04816
Franke-Whittle, I. H., Walter, A., Ebner, C., and Insam, H. (2014). Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 34, 2080–2089. doi: 10.1016/j.wasman.2014.07.020
Fuchs, W., and Drosg, B. (2013). Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 67, 1984–1993. doi: 10.2166/wst.2013.075
Garg, R. N., Pathak, H., Das, D. K., and Tomar, R. K. (2005). Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Env. Monit Assess 107, 1–9. doi: 10.1007/s10661-005-2021-x
Ghafoori, E., Flynn, P., and Feddes, J. (2007). Pipeline vs. truck transport of beef cattle manure. Biomass Bioenergy 31, 168–175. doi: 10.1016/j.biombioe.2006.07.007
Goberna, M., Podmirseg, S. M., Waldhuber, S., Knapp, B. A., García, C., and Insam, H. (2011). Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 49, 18–25. doi: 10.1016/j.apsoil.2011.07.007
Harter, T., Lund, J., Darby, J., Fogg, G. E., Howitt, R., Jessoe, K. K., et al. (2012). Addressing Nitrate in California's Drinking Water. UCDAVIS Center for Watershed Sciences.
Hartz, T., and Hanson, B. (2009). Drip Irrigation and Fertigation Management of Processing Tomato. Vegetable Research and Information Center.
Hartz, T. K., and Bottoms, T. G. (2009). Nitrogen requirements of drip-irrigated processing tomatoes. HortScience 44, 1988–1993. doi: 10.21273/HORTSCI.44.7.1988
Hartz, T. K., Johnstone, P. R., Francis, D. M., and Miyao, E. M. (2005). Processing tomato yield and fruit quality improved with potassium fertigation. HortScience 40, 1862. doi: 10.21273/HORTSCI.40.6.1862
Hartz, T. K., Smith, R., and Gaskell, M. (2010). Nitrogen availability from liquid organic fertilizers. Horttechnology 20, 169–172. doi: 10.21273/HORTTECH.20.1.169
Insam, H., Gómez-Brandón, M., and Ascher, J. (2015). Manure-based biogas fermentation residues - friend or foe of soil fertility? Soil Biol. Biochem. 84, 1–14. doi: 10.1016/j.soilbio.2015.02.006
Johnstone, P. R., Hartz, T. K., LeStrange, M., Nunez, J. J., and Miyao, E. M. (2005). Managing fruit soluble solids with late-season deficit irrigation in drip-irrigated processing tomato production. HortScience 40, 1857–1861. doi: 10.21273/HORTSCI.40.6.1857
Lili, W., Wenzhe, L., Zhongjiang, W., Zhiwu, W., Chao, S., and Yan, L. (2016). Effects of digestate application depth on soil nitrogen volatilization and vertical distribution. Int. J. Agric. Biol. Eng. 9, 101–107. doi: 10.3965/j.ijabe.20160905.2396
Mangiafico, S. S. (2016). Summary and Analysis of Extension Program Evaluation in R, Version 1.18.1: Transforming Data. New Brunswick, NJ.
Maucieri, C., Nicoletto, C., Caruso, C., Sambo, P., and Borin, M. (2017). Effects of digestate solid fraction fertilisation on yield and soil carbon dioxide emission in a horticulture succession. Ital. J. Agron. 11. doi: 10.4081/ija.2017.800
Möller, K. (2015). Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 35, 1021–1041. doi: 10.1007/s13593-015-0284-3
Möller, K., and Müller, T. (2012). Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng. Life Sci. 12, 242–257. doi: 10.1002/elsc.201100085
Möller, K., Stinner, W., Deuker, A., and Leithold, G. (2008). Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 82, 209–232. doi: 10.1007/s10705-008-9196-9
Montes, F., Meinen, R., Dell, C., Rotz, A., Hristov, A. N., Oh, J., et al. (2013). SPECIAL TOPICS-mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options. J. Anim. Sci. 91, 5070–5094. doi: 10.2527/jas.2013-6584
Moya, C., Oyanedel, E., Verdugo, G., Flores, M. F., Urrestarazu, M., and Álvaro, J.E. (2017). Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 52, 868–872. doi: 10.21273/hortsci12026-17
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Nkoa, R. (2014). Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron. Sustain. Dev. 34, 473–492. doi: 10.1007/s13593-013-0196-z
Odlare, M., Pell, M., and Svensson, K. (2008). Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 28, 1246–1253. doi: 10.1016/j.wasman.2007.06.005
Pibars, S. K., Mansour, H. A., and Imam, H. M. (2015). Effect of organic manure fertigation on sesame yield productivity under drip irrigation system. Glob. Adv. Res. J. Agric. Sci. 4, 378–386.
Pieper, J. R., and Barrett, D. M. (2009). Effects of organic and conventional production systems on quality and nutritional parameters of processing tomatoes. J. Sci. Food Agric. 89, 177–194. doi: 10.1002/jsfa.3437
Plana, P. V., and Noche, B. (2016). “A review of the current digestate distribution models: storage and transport,” in 8th International Conference on Waste Management and the Environment, eds C. A. Brebbia and H. Itoh (Valencia: WIT Press), 345–357.
Ponce, K. H., Peet, M. M., Harlow, C. D., Cheng, J., and Willits, D. H. (2002). “Assessment of swine waste bioremediation using greenhouse tomatoes,” in 26th International Horticultural Congress. Acta Horticulturae, ed A. P. Papadopoulos (Toronto), 412–423.
Psomopoulos, C. S., Bourka, A., and Themelis, N. J. (2009). Waste-to-energy: a review of the status and benefits in USA. Waste Manag. 29, 1718–1724. doi: 10.1016/j.wasman.2008.11.020
Qi, X., Zhang, S., Wang, Y., and Wang, R. (2005). Advantages of the integrated pig-biogas-vegetable greenhouse system in North China. Ecol. Eng. 24, 175–183. doi: 10.1016/j.ecoleng.2004.11.001
Renquist, A. R., and Reid, J. B. (1998). Quality of processing tomato (Lycoperscion esculentum) fruit from four bloom dates in relation to optimal harvest timing. N. Z. J. Crop Hortic. Sci. 26, 161–168. doi: 10.1080/01140671.1998.9514052
Rodríguez, F., Pedreschi, R., Fuentealba, C., de Kartzow, A., Olaeta, J. A., and Alvaro, J. E. (2019). The increase in electrical conductivity of nutrient solution enhances compositional and sensory properties of tomato fruit cv. Patrón. Sci. Hortic. 244, 388–398. doi: 10.1016/j.scienta.2018.09.059
Rouphael, Y., Raimondi, G., Caputo, R., and De Pascale, S. (2019). Fertigation strategies for improving water use efficiency and limiting nutrient loss in soilless hippeastrum production. HortScience. 51, 684–689. doi: 10.21273/hortsci.51.6.684
Sandoval-Villa, M., Wood, C. W., and Guertal, E. A. (2002). Tomato leaf chlorophyll meter readings as affected by variety, nitrogen form, and nighttime nutrient solution strength. J. Plant Nutr. 25, 2129–2142. doi: 10.1081/PLN-120014065
Sommer, S. G., and Hutchings, N. J. (2001). Ammonia emission from field applied manure and its reduction - invited paper. Eur. J. Agron. 15, 1–15. doi: 10.1016/S1161-0301(01)00112-5
Stoknes, K., Scholwin, F., Krzesinski, W., Wojciechowska, E., and Jasinska, A. (2016). Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 56, 466–476. doi: 10.1016/j.wasman.2016.06.027
Tambone, F., and Adani, F. (2017). Nitrogen mineralization from digestate in comparison to sewage sludge, compost and urea in a laboratory incubated soil experiment. J. Plant Nutr. Soil Sci. 180, 355–365. doi: 10.1002/jpln.201600241
Tigist, M., Workneh, T. S., and Woldetsadik, K. (2013). Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 50, 477–486. doi: 10.1007/s13197-011-0378-0
Tindula, G. N., Orang, M. N., and Snyder, R. L. (2013). Survey of irrigation methods in California in 2010. J. Irrig. Drain. Eng. 134. doi: 10.1061/(ASCE)0733-9437(2008)134:1(96)
Vaneeckhaute, C., Lebuf, V., Michels, E., Belia, E., Vanrolleghem, P. A., Tack, F. M. G., et al. (2016). Nutrient recovery from digestate: systematic technology review and product classification. Waste Biomass Valorization 8, 21–40. doi: 10.1007/s12649-016-9642-x
Vaughn, S. F., Eller, F. J., Evangelista, R. L., Moser, B. R., Lee, E., Wagner, R. E., et al. (2015). Evaluation of biochar-anaerobic potato digestate mixtures as renewable components of horticultural potting media. Ind. Crops Prod. 65, 467–471. doi: 10.1016/j.indcrop.2014.10.040
Vermeulen, S. J., Campbell, B. M., and Ingram, J. S. I. (2012). Climate change and food systems. Annu. Rev. Environ. Resour. 37, 195–222. doi: 10.1146/annurev-environ-020411-130608
Walsh, J. J., Jones, D. L., Edwards-Jones, G., and Williams, A. P. (2012). Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant Nutr. Soil Sci. 175, 840–845. doi: 10.1002/jpln.201200214
Yu, F. B., Luo, X. P., Song, C. F., Zhang, M. X., and Shan, S. D. (2010). Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. B Soil Plant Sci. 60, 262–268. doi: 10.1080/09064710902893385
Keywords: anaerobic digestate, tomatoes, liquid fertilizers, biofertilizer, subsurface drip fertigation, ultrafiltration
Citation: Barzee TJ, Edalati A, El-Mashad H, Wang D, Scow K and Zhang R (2019) Digestate Biofertilizers Support Similar or Higher Tomato Yields and Quality Than Mineral Fertilizer in a Subsurface Drip Fertigation System. Front. Sustain. Food Syst. 3:58. doi: 10.3389/fsufs.2019.00058
Received: 06 April 2019; Accepted: 08 July 2019;
Published: 25 July 2019.
Edited by:
José María De La Rosa, Institute of Natural Resources and Agrobiology of Seville (CSIC), SpainReviewed by:
Maria Luz Cayuela, Spanish National Research Council (CSIC), SpainJuan Fernando Hirzel, Institute of Agricultural Research, Chile
Copyright © 2019 Barzee, Edalati, El-Mashad, Wang, Scow and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyler J. Barzee, dGpiYXJ6ZWVAdWNkYXZpcy5lZHU=; Ruihong Zhang, cmh6aGFuZ0B1Y2RhdmlzLmVkdQ==
 Tyler J. Barzee
Tyler J. Barzee Abdolhossein Edalati
Abdolhossein Edalati Hamed El-Mashad
Hamed El-Mashad Daoyuan Wang
Daoyuan Wang Kate Scow
Kate Scow Ruihong Zhang1*
Ruihong Zhang1*