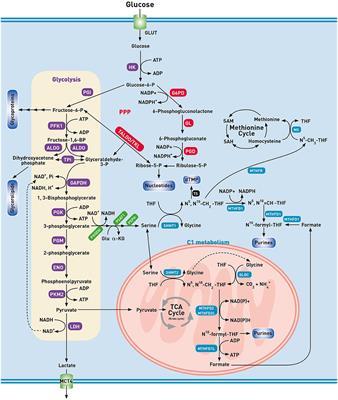
CORRECTION
Published on 12 Apr 2022
Corrigendum: Pathogenetic, Prognostic, and Therapeutic Role of Fatty Acid Synthase in Human Hepatocellular Carcinoma
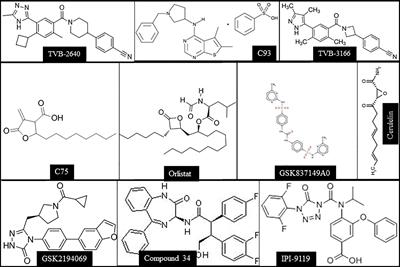
doi 10.3389/fonc.2022.874053
- 646 views
53k
Total downloads
228k
Total views and downloads
CORRECTION
Published on 12 Apr 2022

EDITORIAL
Published on 16 Dec 2020
ORIGINAL RESEARCH
Published on 15 May 2020
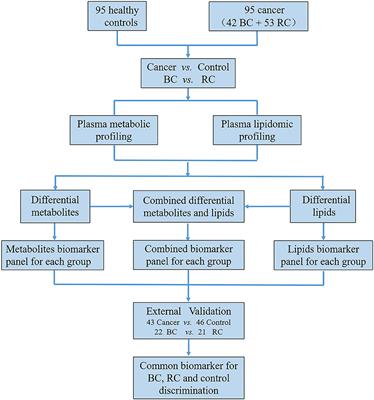
REVIEW
Published on 13 Mar 2020
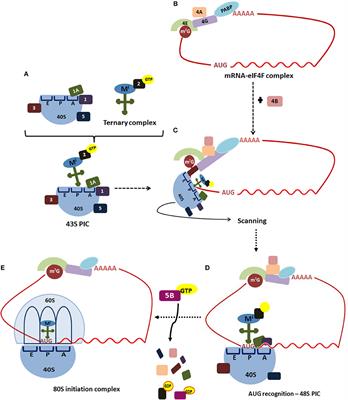
ORIGINAL RESEARCH
Published on 11 Feb 2020
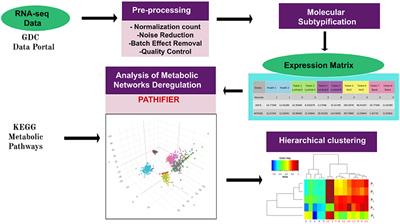
REVIEW
Published on 13 Dec 2019
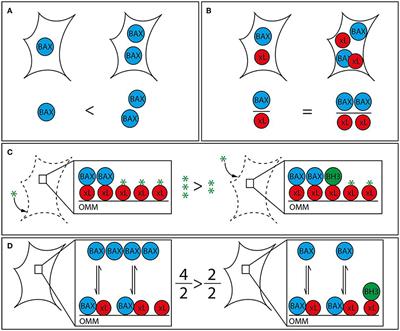
REVIEW
Published on 13 Dec 2019
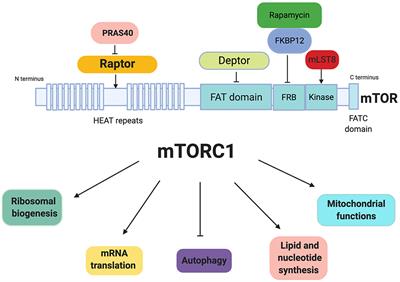
REVIEW
Published on 11 Dec 2019
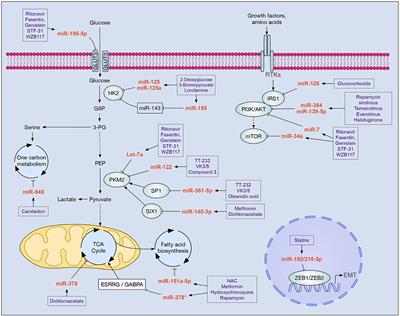
REVIEW
Published on 11 Dec 2019
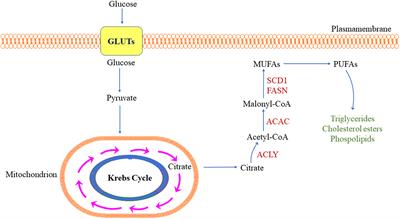
ORIGINAL RESEARCH
Published on 03 Dec 2019
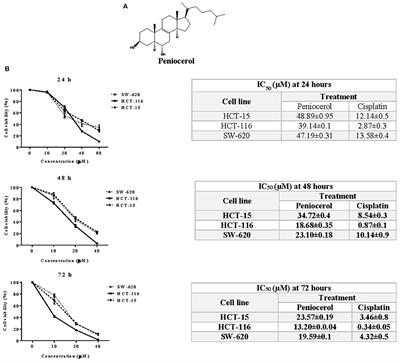
REVIEW
Published on 29 Nov 2019

REVIEW
Published on 15 Nov 2019