MINI REVIEW
Published on 24 Mar 2020
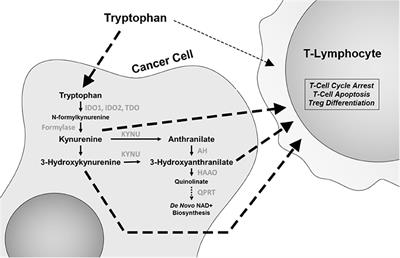
Amino Acid Oncometabolism and Immunomodulation of the Tumor Microenvironment in Lung Cancer
doi 10.3389/fonc.2020.00276
- 5,987 views
- 31 citations
23k
Total downloads
73k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
MINI REVIEW
Published on 24 Mar 2020

REVIEW
Published on 17 Mar 2020

MINI REVIEW
Published on 11 Mar 2020

REVIEW
Published on 18 Feb 2020

REVIEW
Published on 11 Feb 2020

REVIEW
Published on 22 Jan 2020

ORIGINAL RESEARCH
Published on 21 Jan 2020

MINI REVIEW
Published on 10 Dec 2019


Frontiers in Immunology