ORIGINAL RESEARCH
Published on 13 Dec 2019
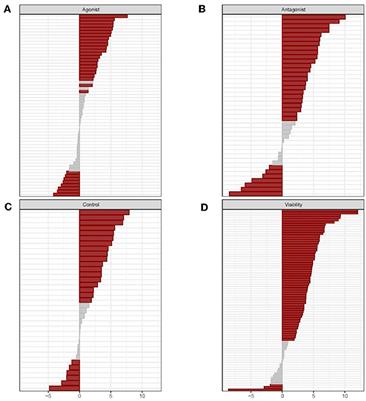
Bioactivity Signatures of Drugs vs. Environmental Chemicals Revealed by Tox21 High-Throughput Screening Assays
doi 10.3389/fdata.2019.00050
- 3,756 views
- 9 citations
12k
Total downloads
76k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
ORIGINAL RESEARCH
Published on 13 Dec 2019

PERSPECTIVE
Published on 11 Dec 2019
REVIEW
Published on 08 Oct 2019
ORIGINAL RESEARCH
Published on 06 Sep 2019

ORIGINAL RESEARCH
Published on 17 Jul 2019
ORIGINAL RESEARCH
Published on 26 Jun 2019

Frontiers in Artificial Intelligence