EDITORIAL
Published on 14 Oct 2021
Editorial: Networks in Movement Disorders. To Move or Not to Move

doi 10.3389/fneur.2021.758246
- 1,200 views
16k
Total downloads
65k
Total views and downloads
EDITORIAL
Published on 14 Oct 2021

REVIEW
Published on 18 Aug 2021

REVIEW
Published on 25 Jun 2021

REVIEW
Published on 03 Jun 2021

ORIGINAL RESEARCH
Published on 25 May 2021

REVIEW
Published on 13 Apr 2021

REVIEW
Published on 08 Apr 2021

PERSPECTIVE
Published on 30 Mar 2021
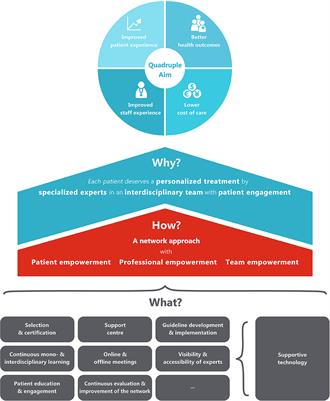
REVIEW
Published on 22 Feb 2021
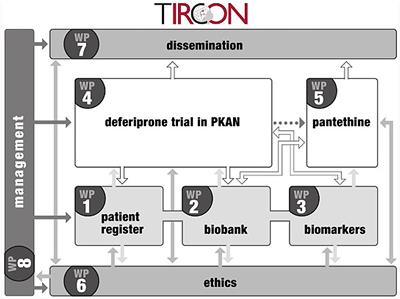
REVIEW
Published on 14 Jan 2021

MINI REVIEW
Published on 26 Aug 2020

ORIGINAL RESEARCH
Published on 26 Jun 2020
