EDITORIAL
Published on 22 May 2020
Editorial: Heparin and Related Polysaccharides
doi 10.3389/fmed.2020.00211
- 2,188 views
- 2 citations
8,065
Total downloads
52k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 22 May 2020
ORIGINAL RESEARCH
Published on 10 Jan 2020

HYPOTHESIS AND THEORY
Published on 15 Nov 2019

ORIGINAL RESEARCH
Published on 27 Jun 2019

ORIGINAL RESEARCH
Published on 18 Apr 2019

MINI REVIEW
Published on 05 Feb 2019

ORIGINAL RESEARCH
Published on 09 Jan 2019
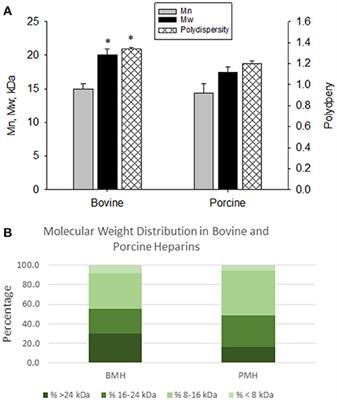
ORIGINAL RESEARCH
Published on 18 Dec 2018
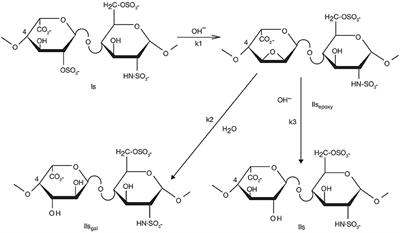
REVIEW
Published on 04 Dec 2018

