EDITORIAL
Published on 13 Nov 2019
Editorial: Drug Re-purposing for the Treatment of Bacterial and Viral Infections
doi 10.3389/fcimb.2019.00387
- 3,910 views
- 1 citation
42k
Total downloads
199k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 13 Nov 2019
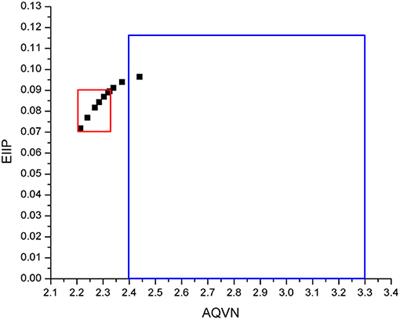
ORIGINAL RESEARCH
Published on 02 Jul 2019
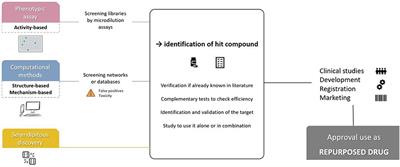
REVIEW
Published on 11 Jun 2019
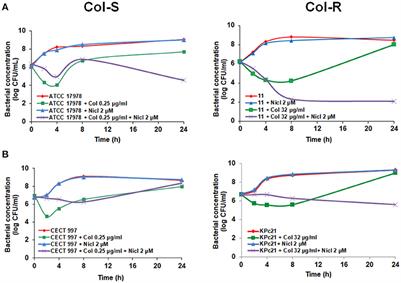
ORIGINAL RESEARCH
Published on 26 Mar 2019
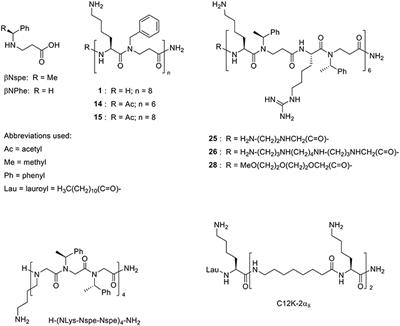
ORIGINAL RESEARCH
Published on 28 Feb 2019
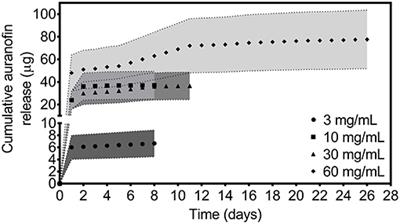
REVIEW
Published on 18 Feb 2019
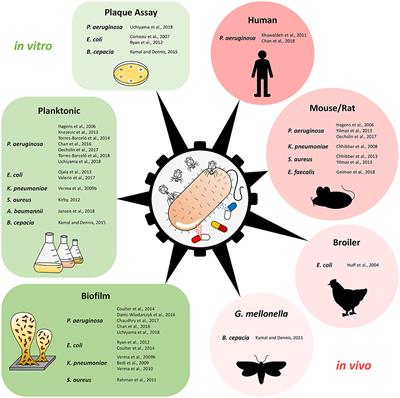
ORIGINAL RESEARCH
Published on 04 Jan 2019
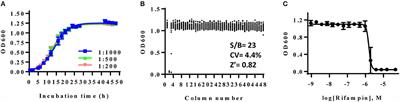
ORIGINAL RESEARCH
Published on 14 Nov 2018
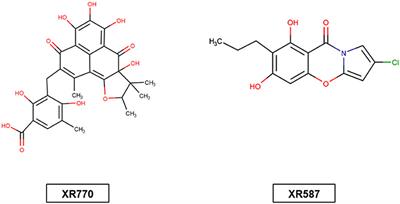
MINI REVIEW
Published on 23 Oct 2018
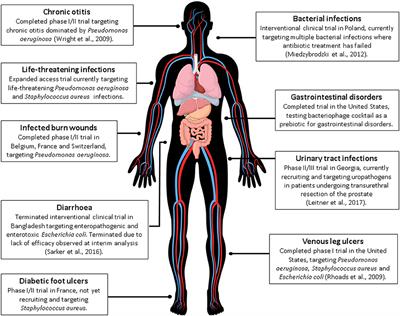
ORIGINAL RESEARCH
Published on 23 Oct 2018
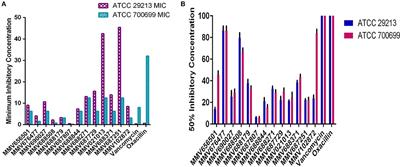
ORIGINAL RESEARCH
Published on 18 Oct 2018
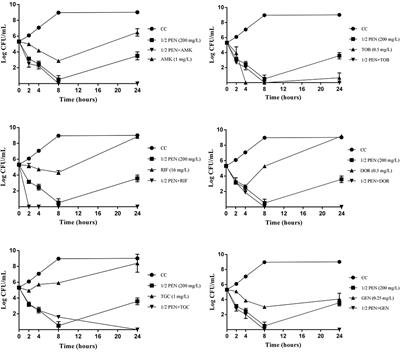
ORIGINAL RESEARCH
Published on 03 Oct 2018