EDITORIAL
Published on 17 May 2019
Editorial: Difficult and Severe Asthma in Children
doi 10.3389/fped.2019.00205
- 2,608 views
- 3 citations
50k
Total downloads
237k
Total views and downloads
EDITORIAL
Published on 17 May 2019
REVIEW
Published on 19 Mar 2019
REVIEW
Published on 11 Feb 2019

REVIEW
Published on 16 Jan 2019

REVIEW
Published on 03 Oct 2018

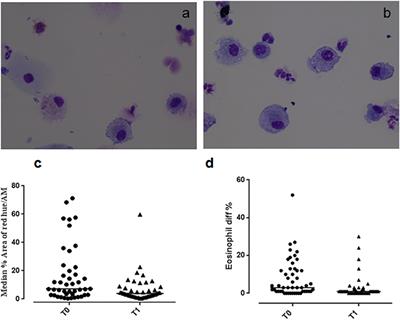
ORIGINAL RESEARCH
Published on 20 Sep 2018

REVIEW
Published on 20 Sep 2018

REVIEW
Published on 23 Aug 2018

SYSTEMATIC REVIEW
Published on 21 Aug 2018

MINI REVIEW
Published on 21 Aug 2018

ORIGINAL RESEARCH
Published on 02 Aug 2018
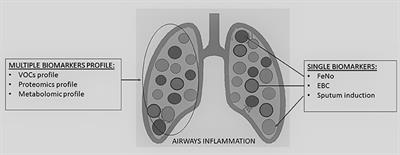
MINI REVIEW
Published on 06 Jul 2018