EDITORIAL
Published on 20 Nov 2018
Editorial: Current Perspectives on Insulin-Like Growth Factor Binding Protein (IGFBP) Research
doi 10.3389/fendo.2018.00667
- 1,225 views
- 4 citations
24k
Total downloads
139k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 20 Nov 2018
ORIGINAL RESEARCH
Published on 15 Oct 2018

CORRECTION
Published on 04 Sep 2018
REVIEW
Published on 30 Aug 2018

REVIEW
Published on 16 Jul 2018

ORIGINAL RESEARCH
Published on 12 Jun 2018
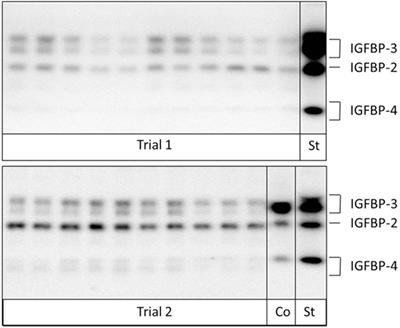
MINI REVIEW
Published on 13 Apr 2018

REVIEW
Published on 09 Apr 2018
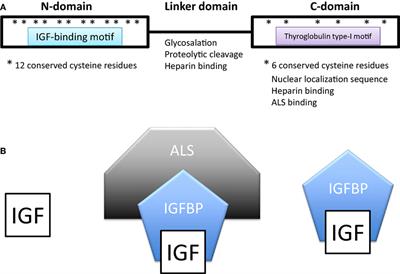
REVIEW
Published on 29 Mar 2018
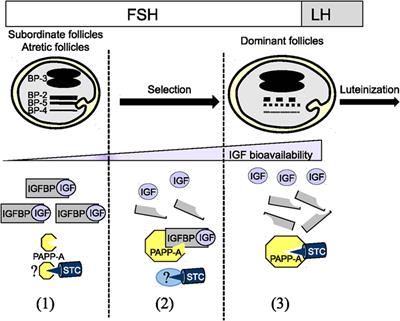
ORIGINAL RESEARCH
Published on 22 Mar 2018

REVIEW
Published on 12 Mar 2018
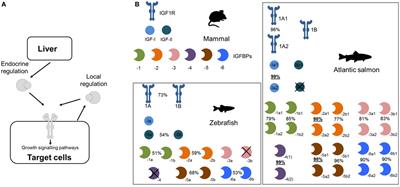
REVIEW
Published on 20 Feb 2018

