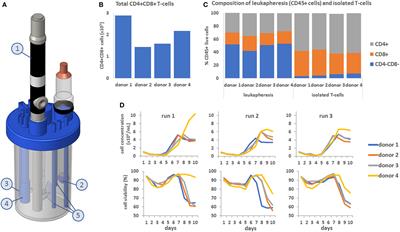
ORIGINAL RESEARCH
Published on 01 Mar 2019
Ex vivo Manufactured Neutrophils for Treatment of Neutropenia—A Process Economic Evaluation
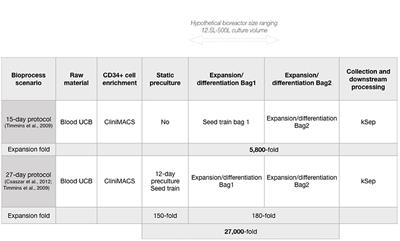
doi 10.3389/fmed.2019.00021
- 7,147 views
- 17 citations
19k
Total downloads
108k
Total views and downloads
ORIGINAL RESEARCH
Published on 01 Mar 2019

MINI REVIEW
Published on 22 Aug 2018
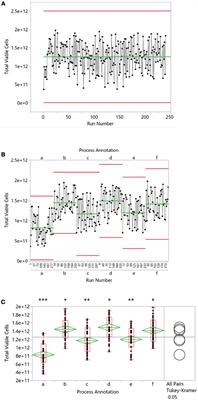
REVIEW
Published on 21 Jun 2018
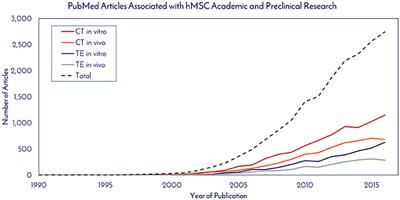
REVIEW
Published on 23 May 2018
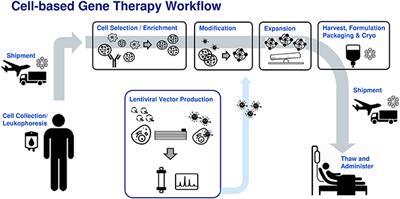
OPINION
Published on 30 Apr 2018
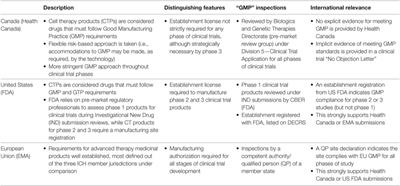
REVIEW
Published on 24 Apr 2018
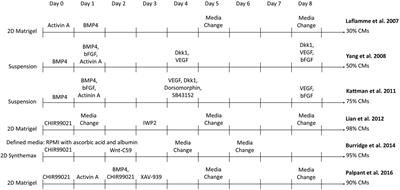
ORIGINAL RESEARCH
Published on 15 Mar 2018
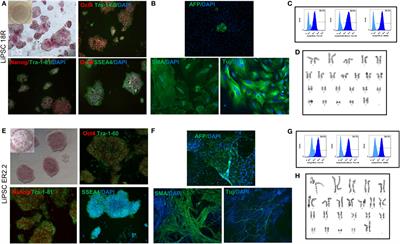
ORIGINAL RESEARCH
Published on 05 Mar 2018