EDITORIAL
Published on 12 Aug 2024
Editorial: Diabetes and cardiovascular disease: new therapeutic interventions
doi 10.3389/fphar.2024.1472636
- 572 views
6,860
Total downloads
21k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 12 Aug 2024
ORIGINAL RESEARCH
Published on 30 Jul 2024

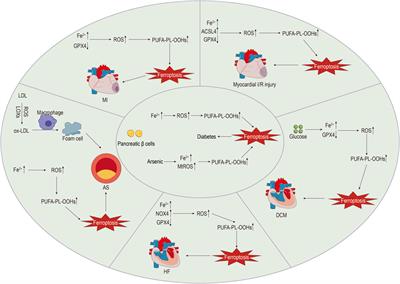
REVIEW
Published on 14 Jun 2024
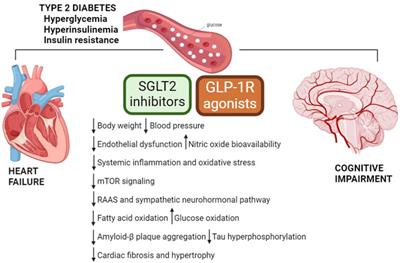
OPINION
Published on 12 Jun 2024
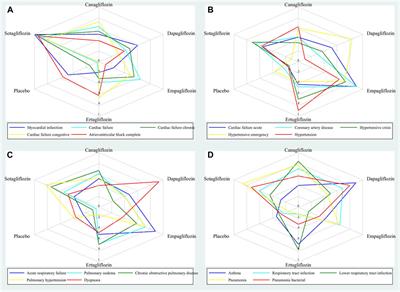
ORIGINAL RESEARCH
Published on 05 Jun 2024
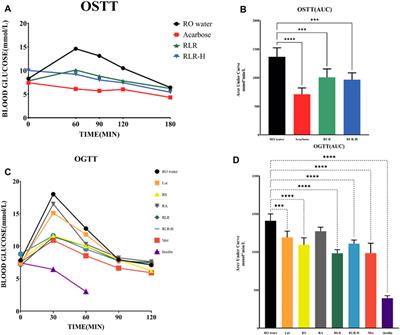
ORIGINAL RESEARCH
Published on 31 May 2024
ORIGINAL RESEARCH
Published on 21 May 2024
ORIGINAL RESEARCH
Published on 10 May 2024
REVIEW
Published on 06 Feb 2024
ORIGINAL RESEARCH
Published on 18 Dec 2023
REVIEW
Published on 26 Oct 2023