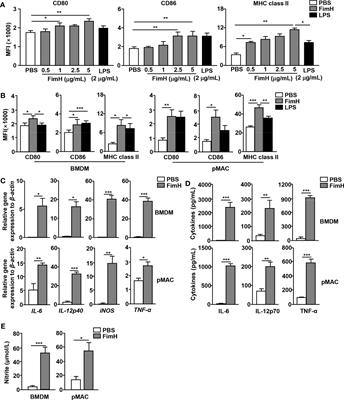
REVIEW
Published on 19 Jun 2024
Fibroblast growth factor signaling in macrophage polarization: impact on health and diseases
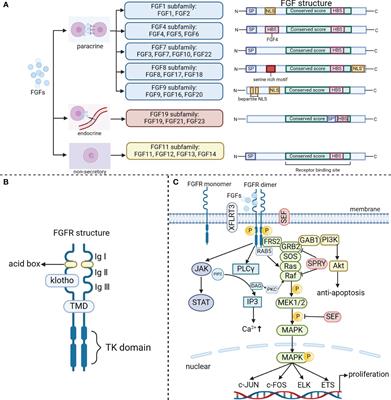
doi 10.3389/fimmu.2024.1390453
- 2,268 views
- 4 citations
5,353
Total downloads
15k
Total views and downloads
You will be redirected to our submission process.
REVIEW
Published on 19 Jun 2024

MINI REVIEW
Published on 05 Apr 2024
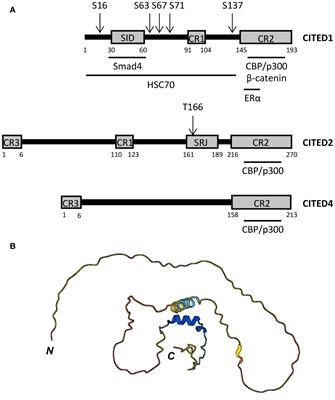
MINI REVIEW
Published on 22 Feb 2024
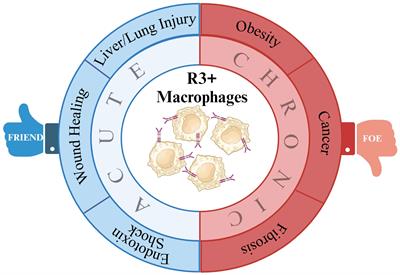
ORIGINAL RESEARCH
Published on 04 Oct 2023
ORIGINAL RESEARCH
Published on 01 Sep 2023