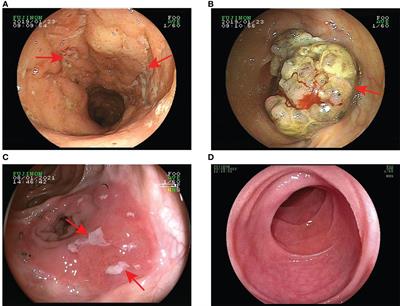
EDITORIAL
Published on 10 Jan 2025
Editorial: Biologic drugs in immune-mediated inflammatory diseases, validation, drug-utilization, effectiveness, regulation, costs, and safety in the real-world
doi 10.3389/fphar.2024.1542453
- 364 views
5,601
Total downloads
26k
Total views and downloads
EDITORIAL
Published on 10 Jan 2025
ORIGINAL RESEARCH
Published on 16 Oct 2024
SYSTEMATIC REVIEW
Published on 10 Oct 2024
REVIEW
Published on 24 Jul 2024
ORIGINAL RESEARCH
Published on 14 Jun 2024
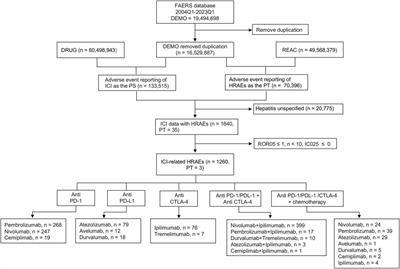
ORIGINAL RESEARCH
Published on 10 Jun 2024

ORIGINAL RESEARCH
Published on 14 Nov 2023

ORIGINAL RESEARCH
Published on 30 Oct 2023
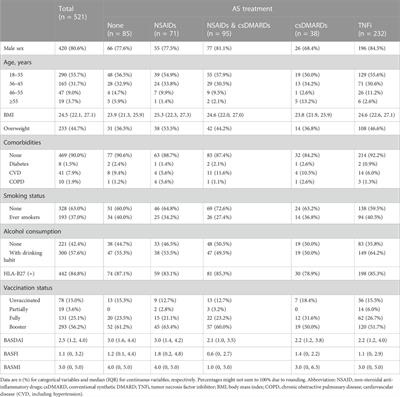
ORIGINAL RESEARCH
Published on 25 Sep 2023
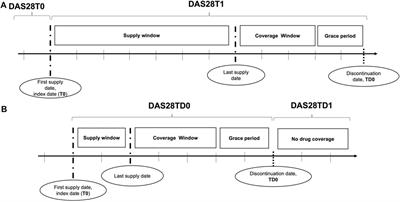
CASE REPORT
Published on 23 May 2023