ORIGINAL RESEARCH
Published on 04 Apr 2024
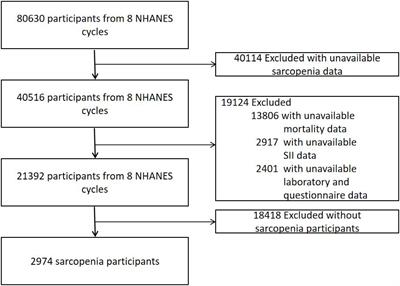
Systemic immune-inflammation index and all-cause and cause-specific mortality in sarcopenia: a study from National Health and Nutrition Examination Survey 1999-2018
doi 10.3389/fimmu.2024.1376544
- 3,640 views
- 6 citations