EDITORIAL
Published on 08 Jul 2024
Editorial: Current therapeutic approaches in Alzheimer's disease: the use of a second drug with anti-amyloid-beta and beyond
doi 10.3389/fnins.2024.1449365
- 731 views
6,940
Total downloads
22k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 08 Jul 2024
BRIEF RESEARCH REPORT
Published on 10 Jun 2024

REVIEW
Published on 30 May 2024
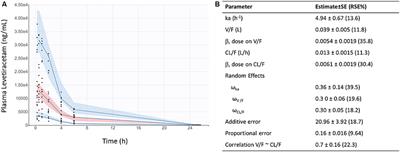
ORIGINAL RESEARCH
Published on 22 May 2024
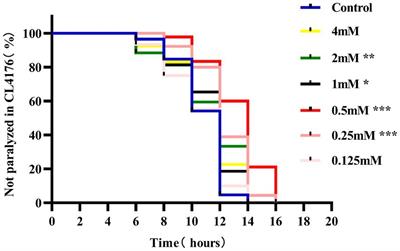
ORIGINAL RESEARCH
Published on 24 Jan 2024
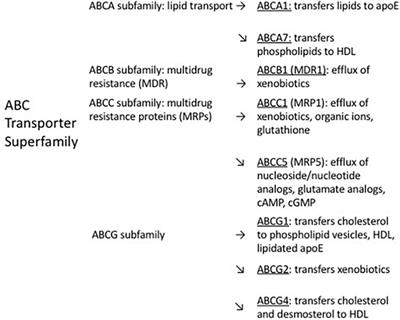
MINI REVIEW
Published on 19 Jan 2024
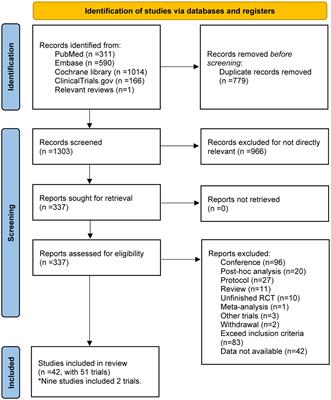
SYSTEMATIC REVIEW
Published on 06 Nov 2023