ORIGINAL RESEARCH
Published on 09 Apr 2024
A disproportionality analysis of FDA adverse event reporting system (FAERS) events for ticagrelor
doi 10.3389/fphar.2024.1251961
- 3,206 views
- 1 citation
13k
Total downloads
51k
Total views and downloads
ORIGINAL RESEARCH
Published on 09 Apr 2024

ORIGINAL RESEARCH
Published on 27 Mar 2024
ORIGINAL RESEARCH
Published on 31 Jan 2024
ORIGINAL RESEARCH
Published on 21 Dec 2023
ORIGINAL RESEARCH
Published on 19 Dec 2023
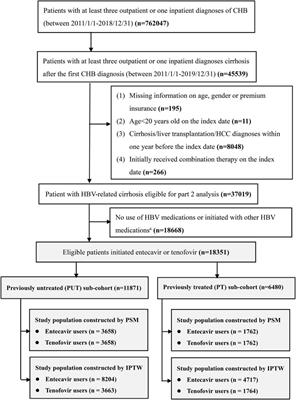
ORIGINAL RESEARCH
Published on 22 Nov 2023
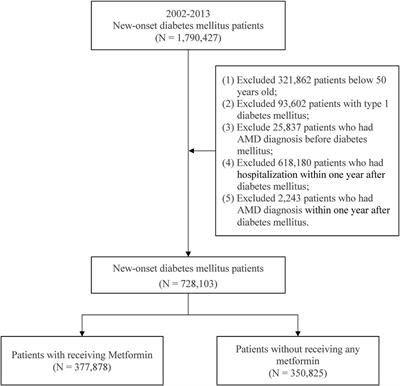
ORIGINAL RESEARCH
Published on 16 Nov 2023
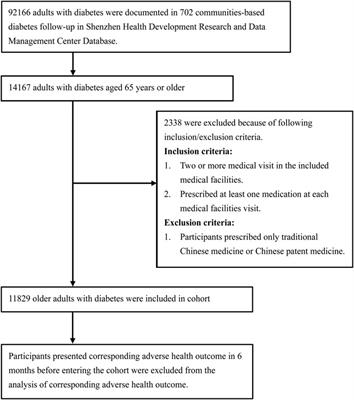
ORIGINAL RESEARCH
Published on 30 Oct 2023
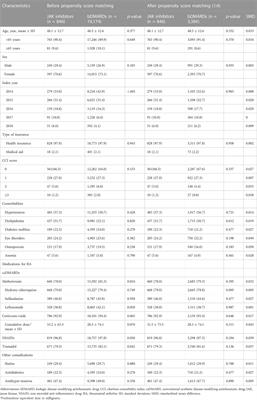
ORIGINAL RESEARCH
Published on 26 Oct 2023
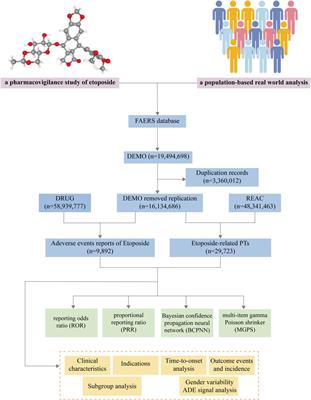
ORIGINAL RESEARCH
Published on 02 Oct 2023

ORIGINAL RESEARCH
Published on 12 Jul 2023
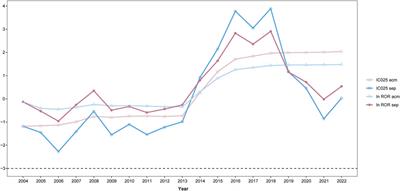
ORIGINAL RESEARCH
Published on 11 Jul 2023
