EDITORIAL
Published on 02 Oct 2018
Editorial: The Role of AAA+ Proteins in Protein Repair and Degradation

doi 10.3389/fmolb.2018.00085
- 10,929 views
- 18 citations
53k
Total downloads
206k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 02 Oct 2018

REVIEW
Published on 24 Aug 2017

MINI REVIEW
Published on 23 Aug 2017

REVIEW
Published on 03 Aug 2017

REVIEW
Published on 19 Jul 2017

REVIEW
Published on 12 Jul 2017

ORIGINAL RESEARCH
Published on 11 Jul 2017

REVIEW
Published on 20 Jun 2017
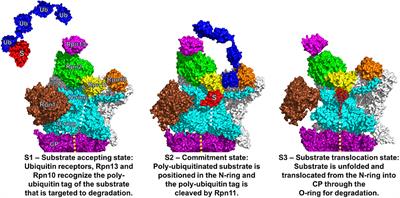
REVIEW
Published on 13 Jun 2017

ORIGINAL RESEARCH
Published on 29 May 2017

REVIEW
Published on 29 May 2017

REVIEW
Published on 22 May 2017

