EDITORIAL
Published on 11 Dec 2023
Editorial: Community series in novel biomarkers for predicting response to cancer immunotherapy: volume II
doi 10.3389/fimmu.2023.1328299
- 463 views
24k
Total downloads
70k
Total views and downloads
EDITORIAL
Published on 11 Dec 2023
STUDY PROTOCOL
Published on 31 Oct 2023
ORIGINAL RESEARCH
Published on 18 Sep 2023
CLINICAL TRIAL
Published on 07 Sep 2023
ORIGINAL RESEARCH
Published on 05 Jun 2023
HYPOTHESIS AND THEORY
Published on 25 May 2023

REVIEW
Published on 22 May 2023

ORIGINAL RESEARCH
Published on 19 May 2023
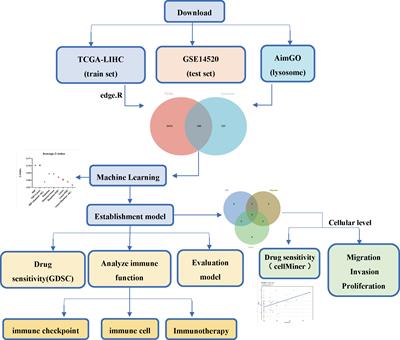
REVIEW
Published on 21 Apr 2023

ORIGINAL RESEARCH
Published on 11 Apr 2023
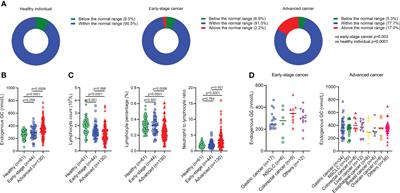
ORIGINAL RESEARCH
Published on 03 Apr 2023
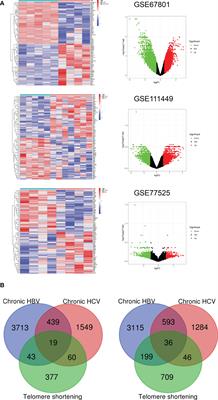
ORIGINAL RESEARCH
Published on 03 Apr 2023