ORIGINAL RESEARCH
Published on 19 Jan 2024
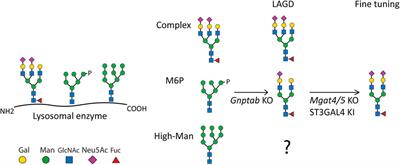
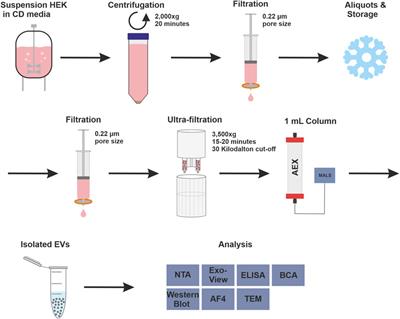
Novel insights into the isolation of extracellular vesicles by anion exchange chromatography
doi 10.3389/fbioe.2023.1298892
- 3,241 views
- 9 citations
4,067
Total downloads
19k
Total views and downloads
ORIGINAL RESEARCH
Published on 19 Jan 2024

ORIGINAL RESEARCH
Published on 17 Jan 2024
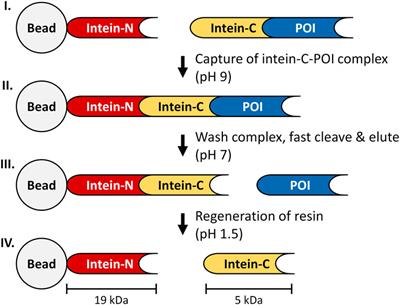
ORIGINAL RESEARCH
Published on 04 Jan 2024
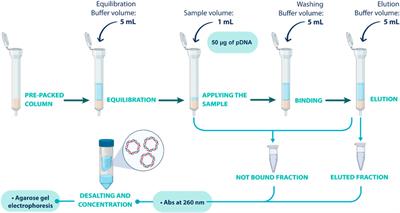
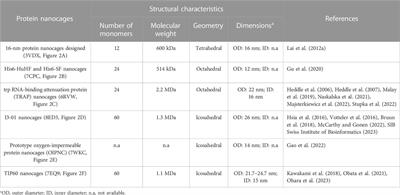
REVIEW
Published on 13 Jul 2023
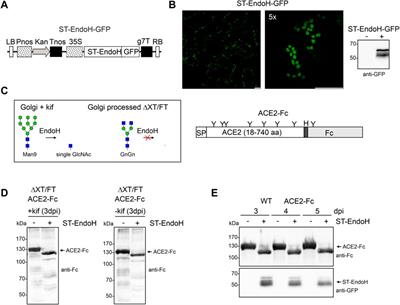
BRIEF RESEARCH REPORT
Published on 03 May 2023
ORIGINAL RESEARCH
Published on 24 Feb 2023