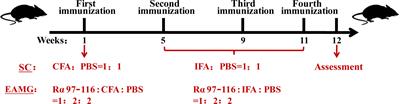
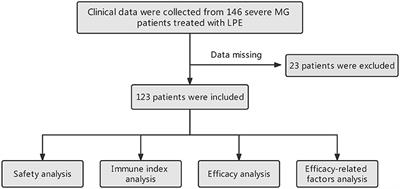
ORIGINAL RESEARCH
Published on 12 Oct 2022
Sorting nexin 17 increases low-density lipoprotein receptor-related protein 4 membrane expression: A novel mechanism of acetylcholine receptor aggregation in myasthenia gravis
doi 10.3389/fimmu.2022.916098
- 2,208 views
- 3 citations