REVIEW
Published on 17 Apr 2023
Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies

doi 10.3389/fimmu.2023.1037945
- 4,059 views
- 15 citations
61k
Total downloads
208k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
REVIEW
Published on 17 Apr 2023

ORIGINAL RESEARCH
Published on 28 Feb 2023
ORIGINAL RESEARCH
Published on 24 Feb 2023

ORIGINAL RESEARCH
Published on 10 Jan 2023

ORIGINAL RESEARCH
Published on 23 Dec 2022
CASE REPORT
Published on 01 Dec 2022

ORIGINAL RESEARCH
Published on 21 Nov 2022

REVIEW
Published on 17 Nov 2022

ORIGINAL RESEARCH
Published on 10 Nov 2022
ORIGINAL RESEARCH
Published on 09 Nov 2022
ORIGINAL RESEARCH
Published on 01 Nov 2022
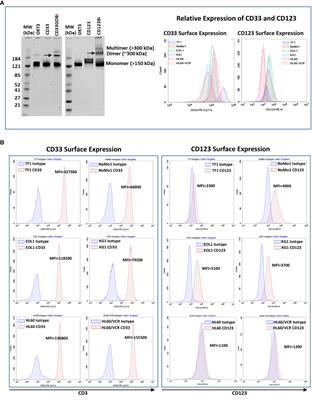
REVIEW
Published on 28 Oct 2022
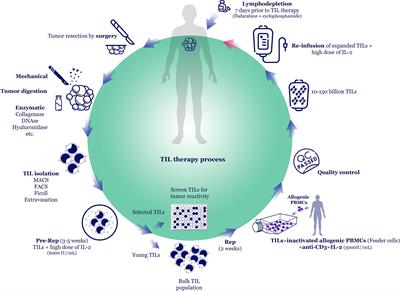

Frontiers in Oncology