EDITORIAL
Published on 12 Oct 2022
Editorial: Shaping with data: Using pharmacoepidemiology to shape pharmaceutical policy and clinical decision-making
doi 10.3389/fphar.2022.1023304
- 802 views
11k
Total downloads
49k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 12 Oct 2022
ORIGINAL RESEARCH
Published on 22 Jun 2022
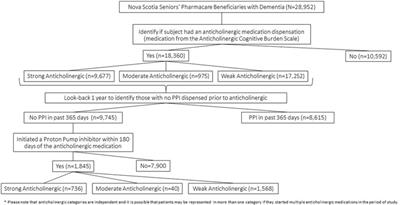
ORIGINAL RESEARCH
Published on 30 May 2022

ORIGINAL RESEARCH
Published on 19 May 2022
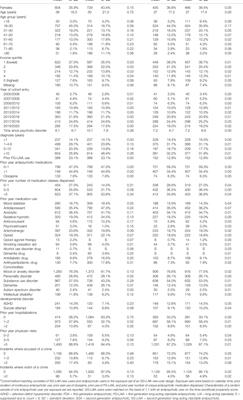
BRIEF RESEARCH REPORT
Published on 04 May 2022
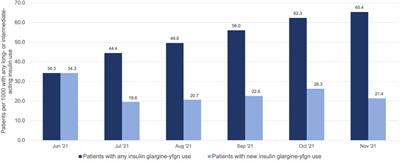
ORIGINAL RESEARCH
Published on 27 Apr 2022

ORIGINAL RESEARCH
Published on 26 Apr 2022

ORIGINAL RESEARCH
Published on 20 Apr 2022
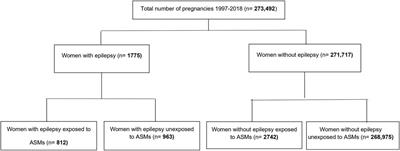
ORIGINAL RESEARCH
Published on 20 Apr 2022
ORIGINAL RESEARCH
Published on 05 Apr 2022
ORIGINAL RESEARCH
Published on 01 Apr 2022
ORIGINAL RESEARCH
Published on 25 Mar 2022

