EDITORIAL
Published on 25 Aug 2022
Editorial: Medicinal Cannabis: Evolution of therapeutic use, future approaches and other implications
doi 10.3389/fphar.2022.999068
- 1,311 views
- 3 citations
26k
Total downloads
205k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 25 Aug 2022
ORIGINAL RESEARCH
Published on 06 Jun 2022
ORIGINAL RESEARCH
Published on 25 May 2022
ORIGINAL RESEARCH
Published on 10 May 2022
MINI REVIEW
Published on 09 May 2022
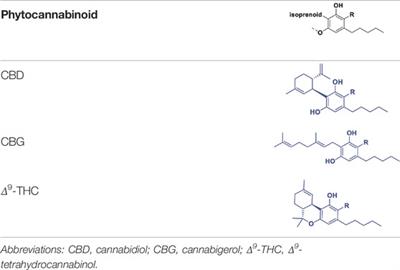
REVIEW
Published on 27 Apr 2022
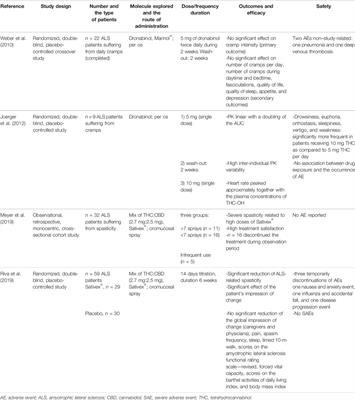
REVIEW
Published on 25 Apr 2022
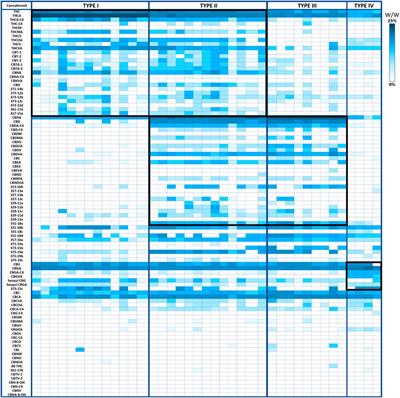
REVIEW
Published on 21 Apr 2022
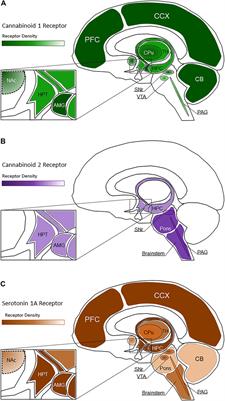
ORIGINAL RESEARCH
Published on 25 Jan 2022
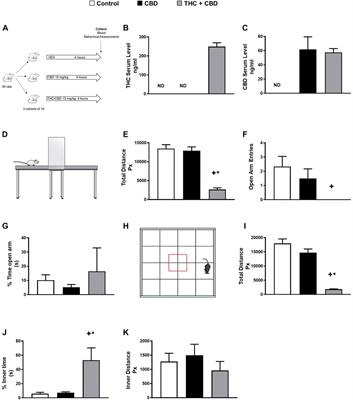
MINI REVIEW
Published on 19 Jan 2022
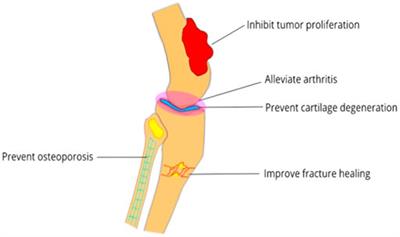
REVIEW
Published on 29 Nov 2021
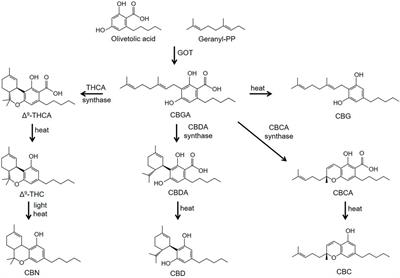
ORIGINAL RESEARCH
Published on 25 Mar 2020