EDITORIAL
Published on 22 Nov 2022
Editorial: Genetically engineered products: Preparing for the future
doi 10.3389/fbioe.2022.1077237
- 873 views
10k
Total downloads
67k
Total views and downloads
EDITORIAL
Published on 22 Nov 2022
ORIGINAL RESEARCH
Published on 07 Oct 2022
POLICY AND PRACTICE REVIEWS
Published on 27 Sep 2022
POLICY AND PRACTICE REVIEWS
Published on 31 Aug 2022
ORIGINAL RESEARCH
Published on 30 Aug 2022
OPINION
Published on 19 Aug 2022
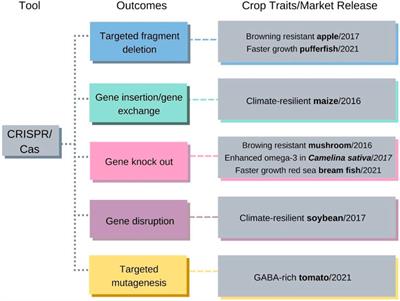
REVIEW
Published on 28 Jun 2022
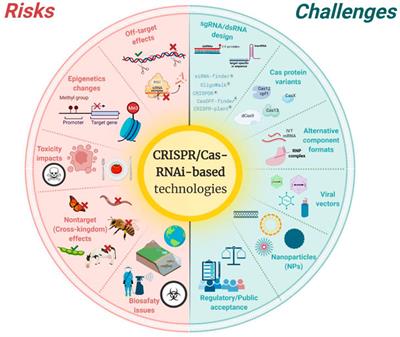
ORIGINAL RESEARCH
Published on 02 May 2022
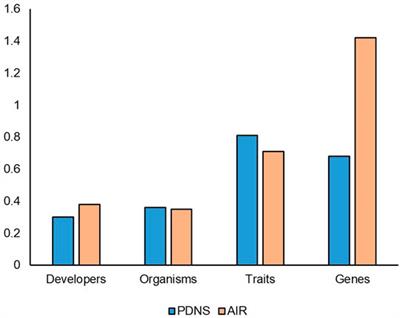
ORIGINAL RESEARCH
Published on 25 Feb 2022
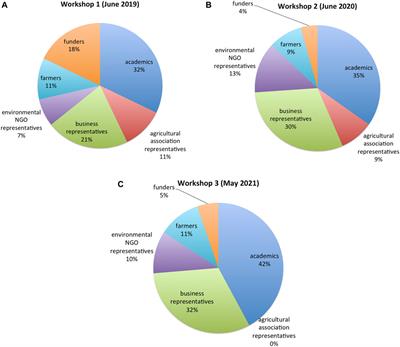
PERSPECTIVE
Published on 21 Feb 2022
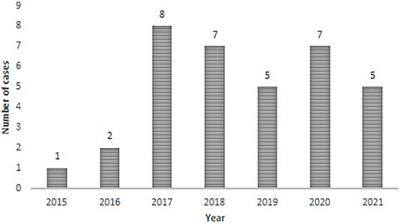
BRIEF RESEARCH REPORT
Published on 18 Feb 2022
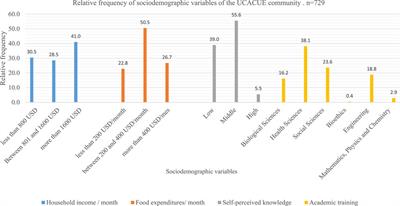
OPINION
Published on 04 Feb 2022
