EDITORIAL
Published on 23 Aug 2023
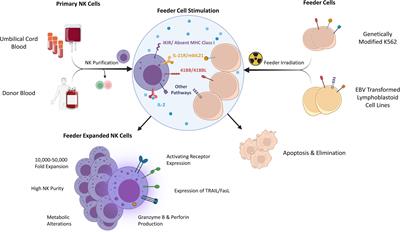
Editorial: Translating NK cell scientific research to clinical product manufacturing
doi 10.3389/fimmu.2023.1229417
- 1,587 views
- 1 citation
28k
Total downloads
90k
Total views and downloads
EDITORIAL
Published on 23 Aug 2023
MINI REVIEW
Published on 08 Jul 2022
ORIGINAL RESEARCH
Published on 01 Jun 2022
ORIGINAL RESEARCH
Published on 07 Apr 2022
ORIGINAL RESEARCH
Published on 07 Apr 2022
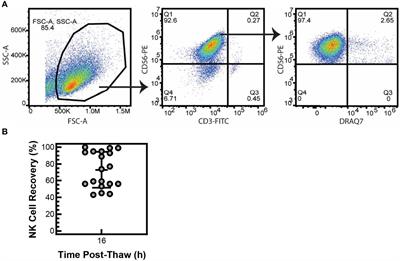
REVIEW
Published on 25 Mar 2022
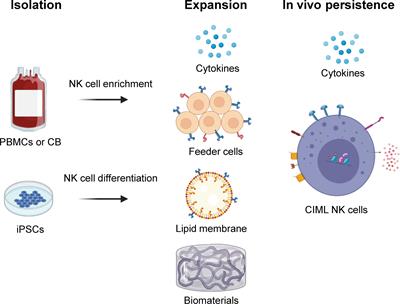
REVIEW
Published on 18 Mar 2022
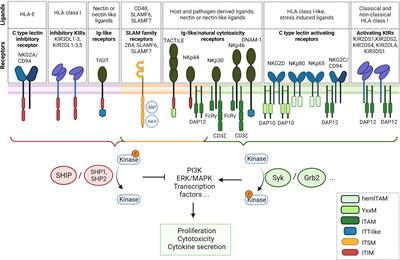
ORIGINAL RESEARCH
Published on 07 Mar 2022
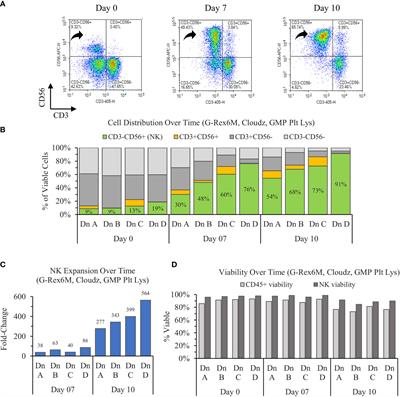
MINI REVIEW
Published on 11 Feb 2022
PERSPECTIVE
Published on 31 Jan 2022