EDITORIAL
Published on 21 Dec 2022
Editorial: Perinatal derivatives and the road to clinical translation, Volume II
doi 10.3389/fbioe.2022.1122728
- 729 views
- 1 citation
16k
Total downloads
53k
Total views and downloads
EDITORIAL
Published on 21 Dec 2022
REVIEW
Published on 08 Nov 2022
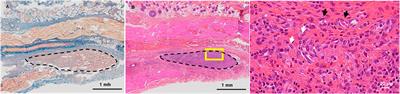
ORIGINAL RESEARCH
Published on 24 Oct 2022
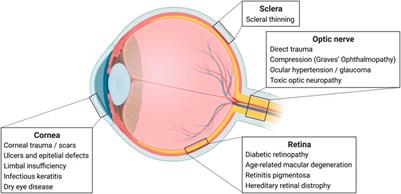
REVIEW
Published on 13 Oct 2022

ORIGINAL RESEARCH
Published on 11 Oct 2022
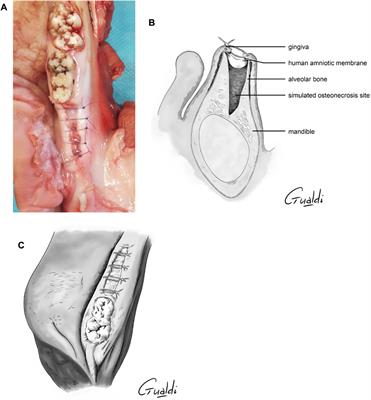
REVIEW
Published on 03 Oct 2022
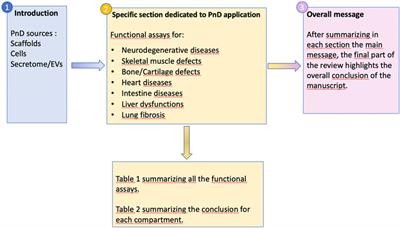
REVIEW
Published on 14 Sep 2022
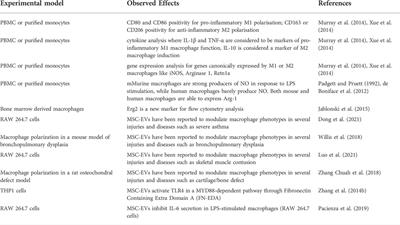
REVIEW
Published on 04 Aug 2022
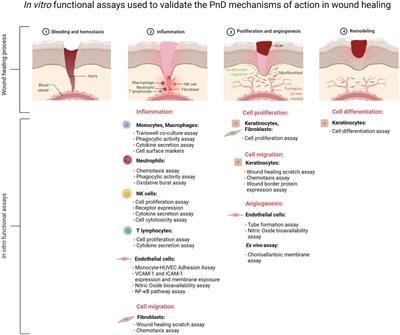
ORIGINAL RESEARCH
Published on 22 Jul 2022
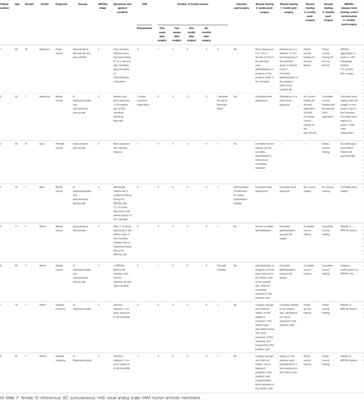
ORIGINAL RESEARCH
Published on 05 Jul 2022
ORIGINAL RESEARCH
Published on 08 Jun 2022
ORIGINAL RESEARCH
Published on 11 Mar 2022