MINI REVIEW
Published on 19 Aug 2022
Potential therapeutics against neurological disorders: Natural products-based drugs
doi 10.3389/fphar.2022.950457
- 8,182 views
- 8 citations
14k
Total downloads
50k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
MINI REVIEW
Published on 19 Aug 2022
EDITORIAL
Published on 16 Aug 2022
ORIGINAL RESEARCH
Published on 26 Jan 2022

SYSTEMATIC REVIEW
Published on 18 Jan 2022

REVIEW
Published on 13 Jan 2022

REVIEW
Published on 09 Dec 2021
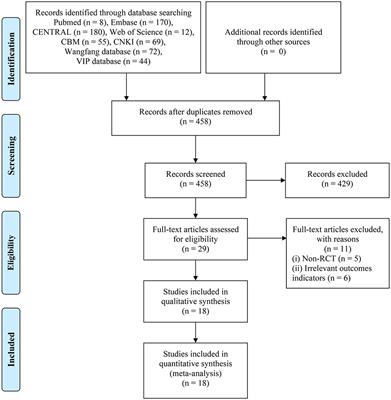
REVIEW
Published on 20 Sep 2021
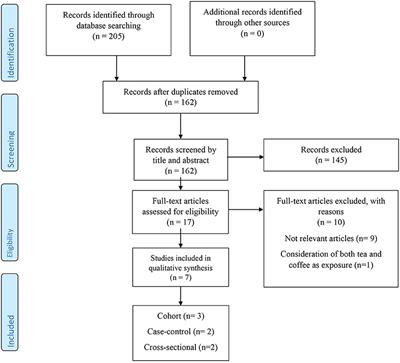
ORIGINAL RESEARCH
Published on 07 Sep 2021

REVIEW
Published on 12 Aug 2021
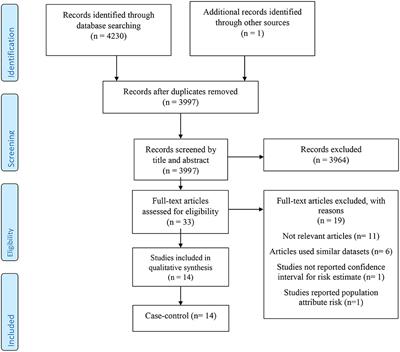
SYSTEMATIC REVIEW
Published on 10 Jun 2021


Frontiers in Nutrition