EDITORIAL
Published on 13 Sep 2022
Editorial: Controversies in growth hormone treatment and diagnosis
doi 10.3389/fendo.2022.1013872
- 1,151 views
22k
Total downloads
103k
Total views and downloads
EDITORIAL
Published on 13 Sep 2022
MINI REVIEW
Published on 24 Aug 2022
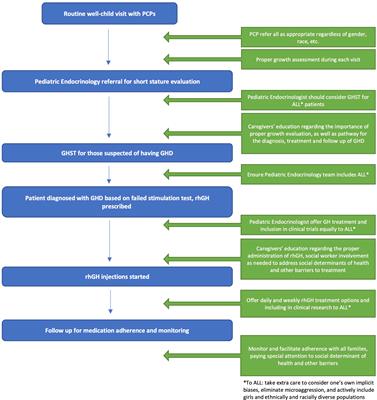
MINI REVIEW
Published on 22 Aug 2022
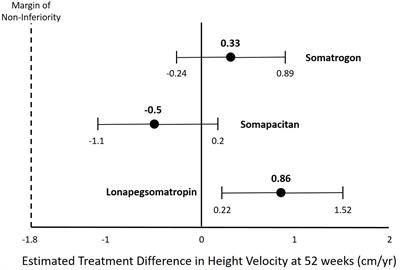
MINI REVIEW
Published on 09 Jun 2022
ORIGINAL RESEARCH
Published on 29 Apr 2022
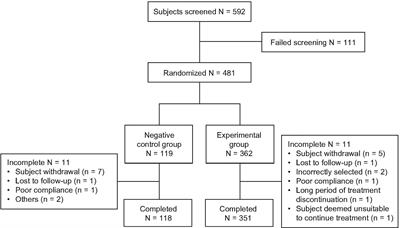
ORIGINAL RESEARCH
Published on 22 Apr 2022
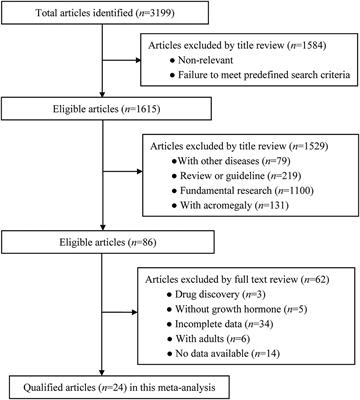
ORIGINAL RESEARCH
Published on 08 Apr 2022
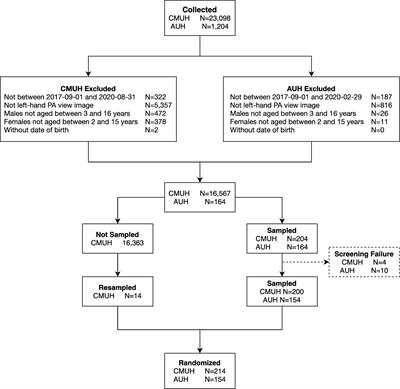
CORRECTION
Published on 06 Apr 2022
SYSTEMATIC REVIEW
Published on 01 Mar 2022
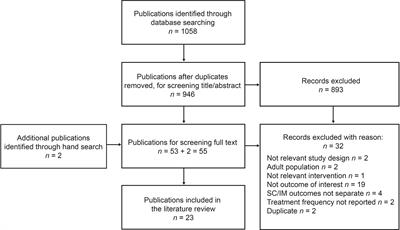
ORIGINAL RESEARCH
Published on 28 Feb 2022
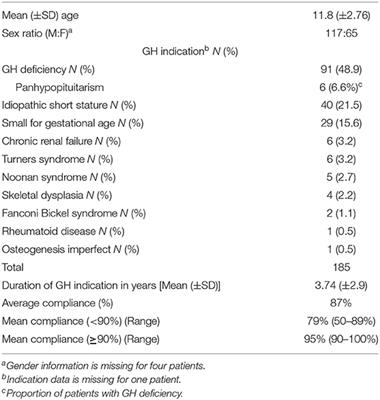
ORIGINAL RESEARCH
Published on 18 Feb 2022
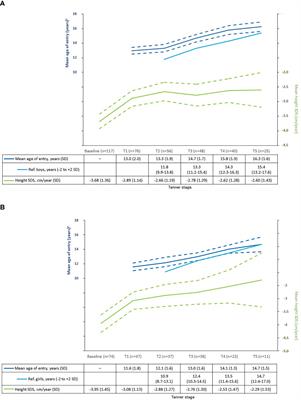
MINI REVIEW
Published on 18 Feb 2022

