ORIGINAL RESEARCH
Published on 15 Jun 2022
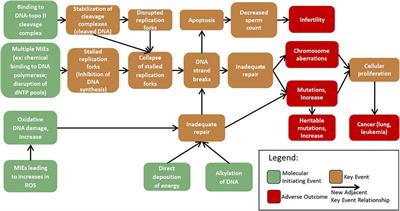
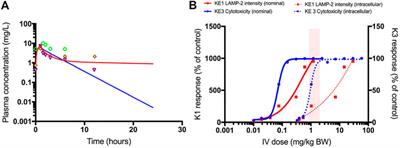
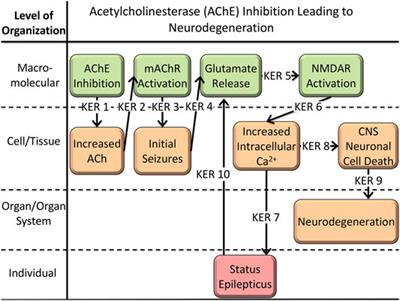
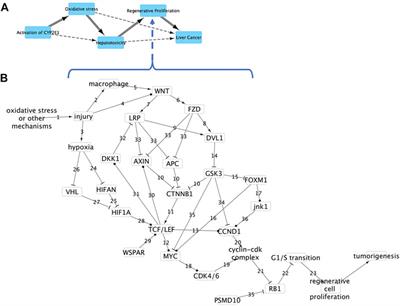
Mapping Adverse Outcome Pathways for Kidney Injury as a Basis for the Development of Mechanism-Based Animal-Sparing Approaches to Assessment of Nephrotoxicity
doi 10.3389/ftox.2022.863643
- 2,595 views
- 4 citations