REVIEW
Published on 23 Mar 2023
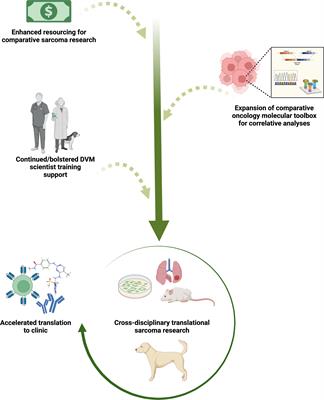
Naturally occurring canine sarcomas: Bridging the gap from mouse models to human patients through cross-disciplinary research partnerships
doi 10.3389/fonc.2023.1130215
- 2,595 views
- 2 citations
5,961
Total downloads
24k
Total views and downloads
REVIEW
Published on 23 Mar 2023

ORIGINAL RESEARCH
Published on 11 Nov 2022

MINI REVIEW
Published on 04 Feb 2022

REVIEW
Published on 20 Oct 2021

ORIGINAL RESEARCH
Published on 08 Oct 2021

ORIGINAL RESEARCH
Published on 02 Apr 2019
