EDITORIAL
Published on 27 Sep 2022
Editorial: Characterization of biotherapeutic products
doi 10.3389/fbioe.2022.993262
- 1,282 views
12k
Total downloads
61k
Total views and downloads
EDITORIAL
Published on 27 Sep 2022
ORIGINAL RESEARCH
Published on 24 May 2022
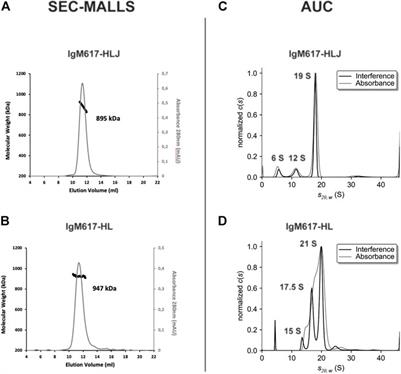
ORIGINAL RESEARCH
Published on 13 Apr 2022
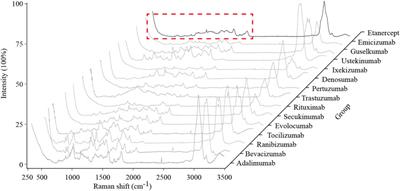
ORIGINAL RESEARCH
Published on 05 Apr 2022

ORIGINAL RESEARCH
Published on 14 Mar 2022
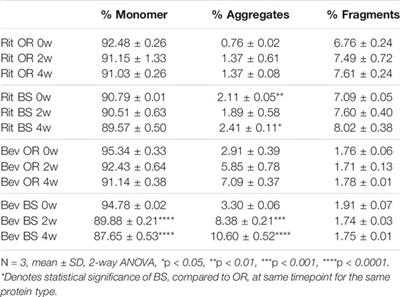
ORIGINAL RESEARCH
Published on 08 Mar 2022
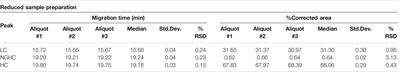
ORIGINAL RESEARCH
Published on 18 Feb 2022
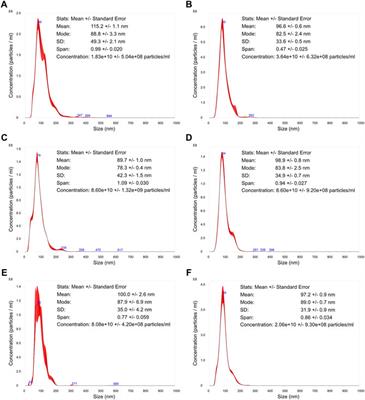
REVIEW
Published on 09 Feb 2022
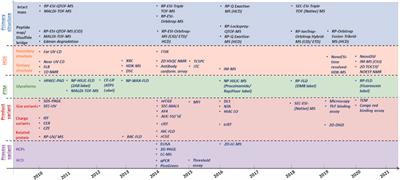
ORIGINAL RESEARCH
Published on 11 Jan 2022
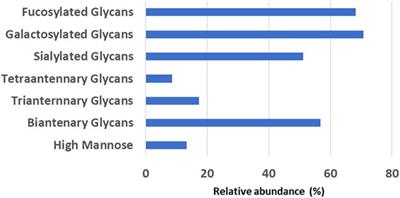
ORIGINAL RESEARCH
Published on 04 Nov 2021

