EDITORIAL
Published on 24 May 2022
Editorial: Therapeutic Drug Monitoring (TDM): A Useful Tool for Pediatric Pharmacology Applied to Routine Clinical Practice
doi 10.3389/fphar.2022.931843
- 3,705 views
- 10 citations
19k
Total downloads
77k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 24 May 2022
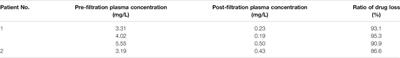
CASE REPORT
Published on 24 Mar 2022
BRIEF RESEARCH REPORT
Published on 18 Mar 2022
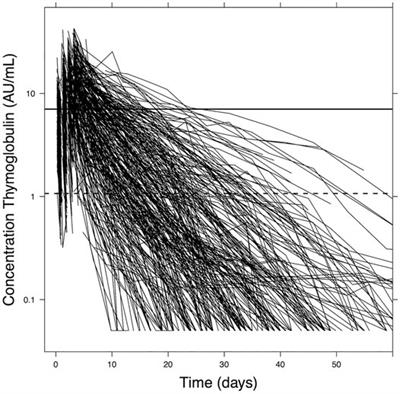
REVIEW
Published on 07 Mar 2022
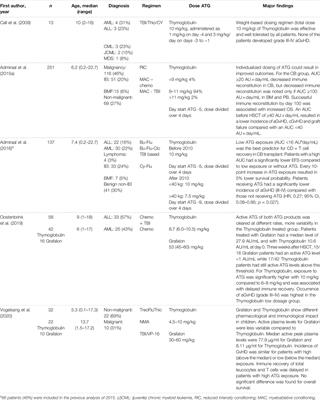
ORIGINAL RESEARCH
Published on 05 Jan 2022
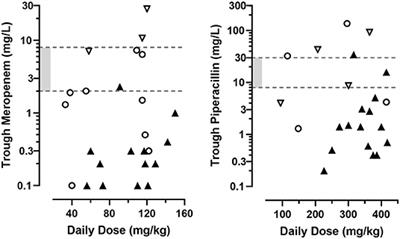
STUDY PROTOCOL
Published on 10 Dec 2021

ORIGINAL RESEARCH
Published on 07 Dec 2021
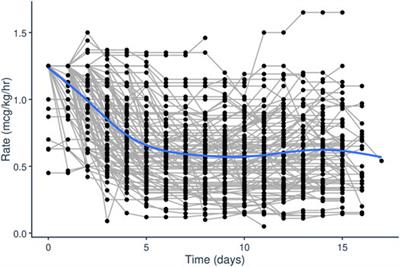
BRIEF RESEARCH REPORT
Published on 06 Dec 2021

BRIEF RESEARCH REPORT
Published on 23 Nov 2021

ORIGINAL RESEARCH
Published on 19 Nov 2021

ORIGINAL RESEARCH
Published on 05 Nov 2021

PERSPECTIVE
Published on 01 Oct 2021


Frontiers in Pediatrics