EDITORIAL
Published on 04 Jul 2022
Editorial: Lessons Learned from Translational Research in Neuromuscular Diseases: Impact on Study Design, Outcome Measures and Managing Expectation
doi 10.3389/fgene.2022.840074
- 1,473 views
- 1 citation
5,073
Total downloads
19k
Total views and downloads
EDITORIAL
Published on 04 Jul 2022
REVIEW
Published on 07 Dec 2021
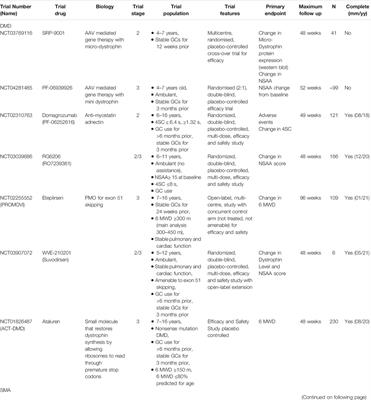
POLICY AND PRACTICE REVIEWS
Published on 10 Nov 2021
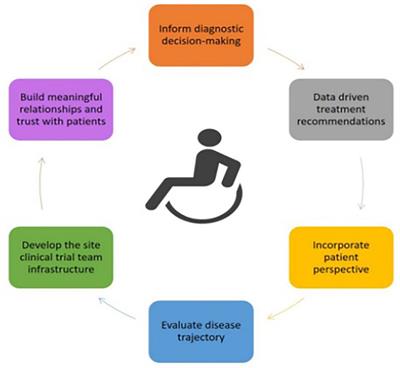
PERSPECTIVE
Published on 01 Nov 2021
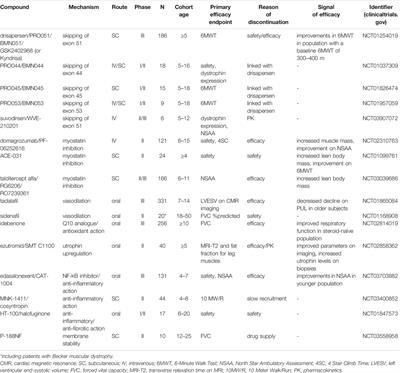
POLICY AND PRACTICE REVIEWS
Published on 29 Oct 2021
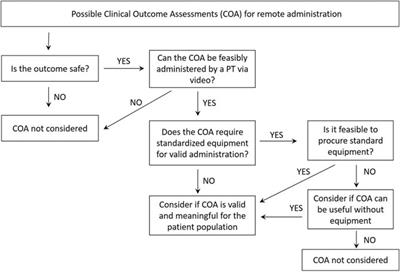
REVIEW
Published on 04 Oct 2021

