EDITORIAL
Published on 11 Jul 2022
Editorial: The Process Evaluation of Clinical Trials
doi 10.3389/fmed.2022.950637
- 2,784 views
- 6 citations
10k
Total downloads
40k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 11 Jul 2022
METHODS
Published on 15 Jun 2022
ORIGINAL RESEARCH
Published on 08 Feb 2022
ORIGINAL RESEARCH
Published on 22 Nov 2021
ORIGINAL RESEARCH
Published on 17 Nov 2021
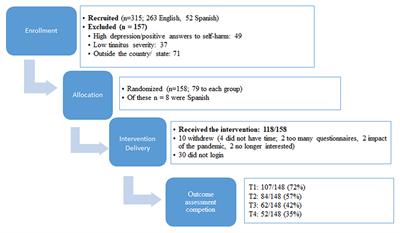
ORIGINAL RESEARCH
Published on 12 Nov 2021
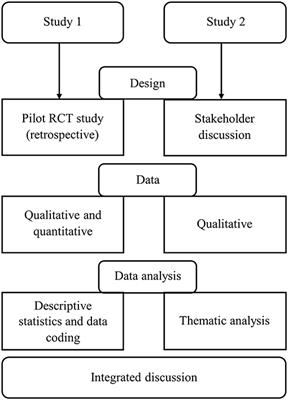
ORIGINAL RESEARCH
Published on 22 Oct 2021
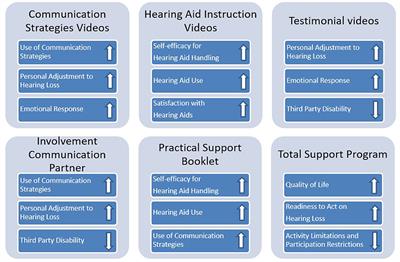
SYSTEMATIC REVIEW
Published on 04 Oct 2021
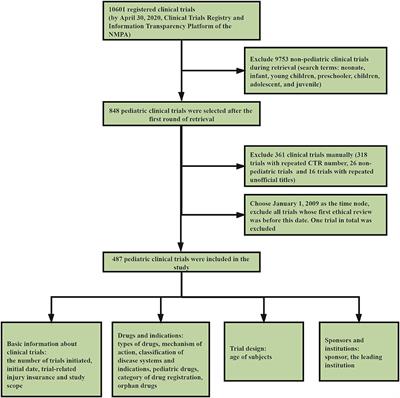
ORIGINAL RESEARCH
Published on 27 Aug 2021
ORIGINAL RESEARCH
Published on 28 May 2021