EDITORIAL
Published on 21 Oct 2022
Editorial: Network pharmacology and traditional medicine: Setting the new standards by combining In silico and experimental work
doi 10.3389/fphar.2022.1002537
- 3,066 views
- 11 citations
76k
Total downloads
210k
Total views and downloads
EDITORIAL
Published on 21 Oct 2022

ORIGINAL RESEARCH
Published on 11 Aug 2022
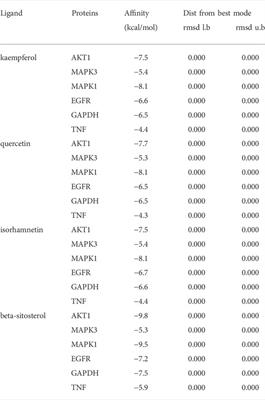
ORIGINAL RESEARCH
Published on 20 Apr 2022
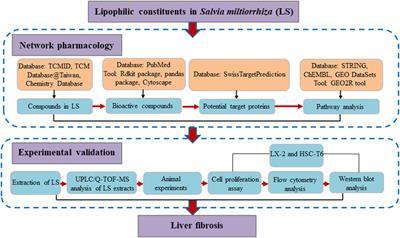
ORIGINAL RESEARCH
Published on 05 Apr 2022
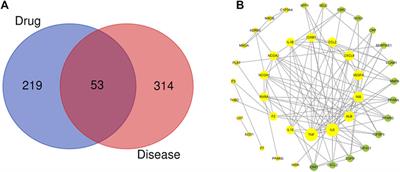
ORIGINAL RESEARCH
Published on 24 Mar 2022
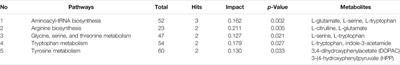
ORIGINAL RESEARCH
Published on 15 Mar 2022
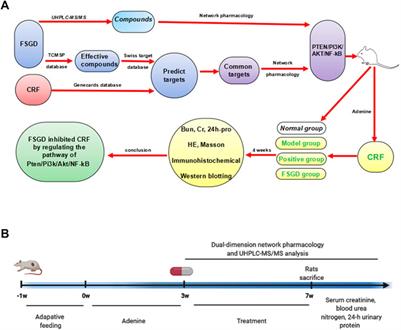
ORIGINAL RESEARCH
Published on 28 Feb 2022

ORIGINAL RESEARCH
Published on 01 Feb 2022
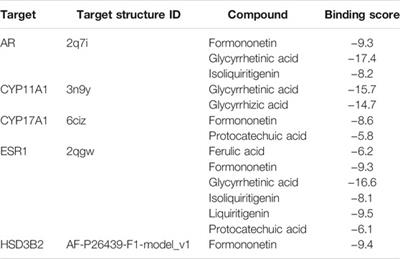
ORIGINAL RESEARCH
Published on 24 Jan 2022
ORIGINAL RESEARCH
Published on 24 Jan 2022
ORIGINAL RESEARCH
Published on 21 Jan 2022

REVIEW
Published on 19 Jan 2022