EDITORIAL
Published on 28 Oct 2021
Editorial: Ubiquitin Code: From Cell Biology to Translational Medicine
doi 10.3389/fcell.2021.791967
- 1,668 views
- 1 citation
30k
Total downloads
97k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 28 Oct 2021
REVIEW
Published on 25 Oct 2021
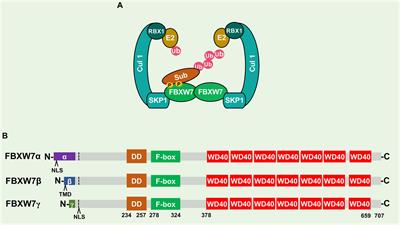
REVIEW
Published on 25 Aug 2021
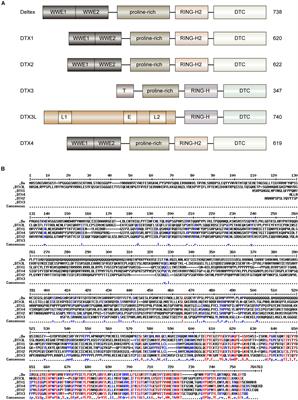
REVIEW
Published on 13 Aug 2021
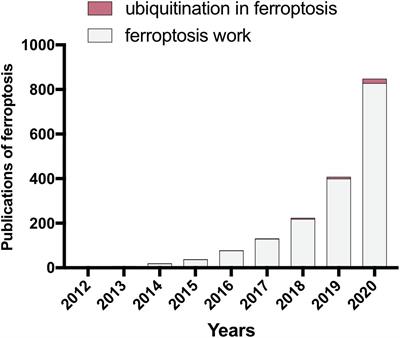
REVIEW
Published on 19 Jul 2021
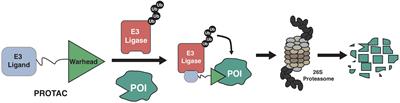
REVIEW
Published on 01 Jul 2021
ORIGINAL RESEARCH
Published on 28 Jun 2021
ORIGINAL RESEARCH
Published on 22 Jun 2021
REVIEW
Published on 07 Jun 2021
ORIGINAL RESEARCH
Published on 07 Jun 2021
REVIEW
Published on 24 May 2021
ORIGINAL RESEARCH
Published on 13 May 2021

