EDITORIAL
Published on 18 Jul 2022
Editorial: Mesenchymal Stromal Cells: Preclinical and Clinical Challenges
doi 10.3389/fcell.2022.969178
- 1,279 views
- 3 citations
43k
Total downloads
170k
Total views and downloads
EDITORIAL
Published on 18 Jul 2022
ORIGINAL RESEARCH
Published on 26 Apr 2022

MINI REVIEW
Published on 13 Apr 2022
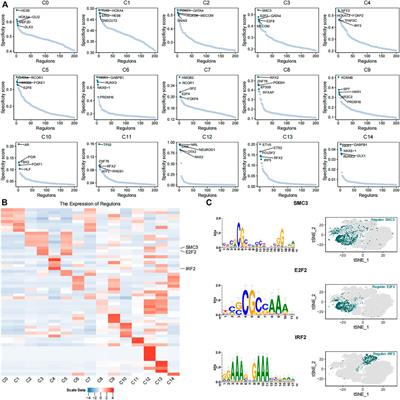
ORIGINAL RESEARCH
Published on 04 Apr 2022
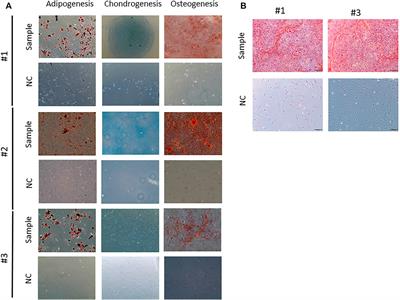
ORIGINAL RESEARCH
Published on 07 Mar 2022
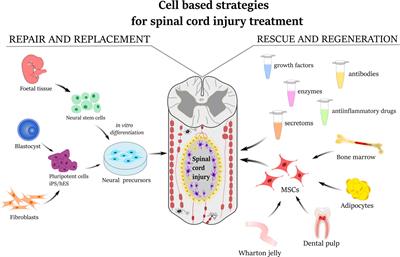
REVIEW
Published on 14 Jan 2022
CORRECTION
Published on 28 Oct 2021
REVIEW
Published on 12 Oct 2021

REVIEW
Published on 10 Sep 2021

ORIGINAL RESEARCH
Published on 04 Aug 2021

REVIEW
Published on 20 Jul 2021

REVIEW
Published on 06 Jul 2021