REVIEW
Published on 29 Sep 2021
Critical Capability Needs for Reduction of Transmission of SARS-CoV-2 Indoors
doi 10.3389/fbioe.2021.641599
- 2,868 views
- 2 citations
25k
Total downloads
169k
Total views and downloads
REVIEW
Published on 29 Sep 2021

ORIGINAL RESEARCH
Published on 06 Sep 2021

EDITORIAL
Published on 24 Aug 2021
ORIGINAL RESEARCH
Published on 24 Jun 2021
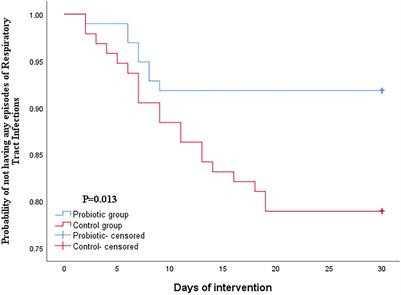
OPINION
Published on 15 Jun 2021

ORIGINAL RESEARCH
Published on 13 May 2021
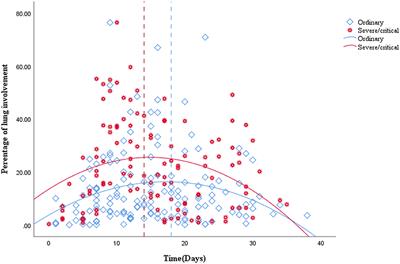
REVIEW
Published on 30 Apr 2021

REVIEW
Published on 14 Apr 2021
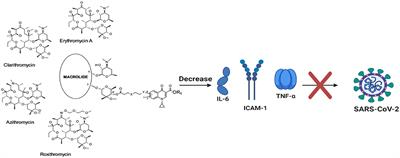
BRIEF RESEARCH REPORT
Published on 29 Mar 2021
BRIEF RESEARCH REPORT
Published on 26 Mar 2021

ORIGINAL RESEARCH
Published on 02 Mar 2021

OPINION
Published on 28 Jan 2021

