EDITORIAL
Published on 13 Apr 2022
Editorial: Modern Approaches to Hemophilia Management: Gene Therapy and Beyond
doi 10.3389/fmed.2022.859710
- 1,117 views
7,840
Total downloads
34k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 13 Apr 2022
CASE REPORT
Published on 11 Feb 2022
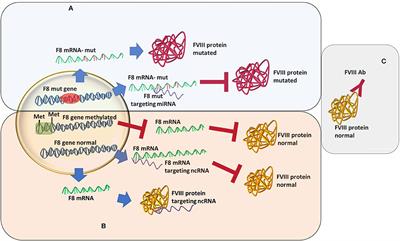
MINI REVIEW
Published on 17 Aug 2021
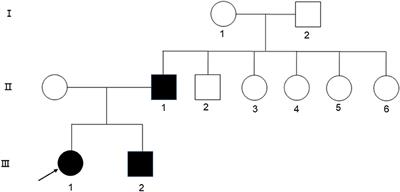
MINI REVIEW
Published on 10 Aug 2021
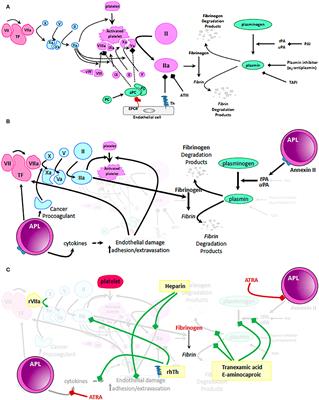
MINI REVIEW
Published on 05 May 2021

MINI REVIEW
Published on 15 Apr 2021