EDITORIAL
Published on 26 Nov 2021
Editorial: Bioethics Amidst the COVID-19 Pandemic
doi 10.3389/fmed.2021.778146
- 2,527 views
- 1 citation
39k
Total downloads
265k
Total views and downloads
EDITORIAL
Published on 26 Nov 2021
PERSPECTIVE
Published on 22 Sep 2021

ORIGINAL RESEARCH
Published on 26 Jul 2021

SYSTEMATIC REVIEW
Published on 07 Jul 2021

ORIGINAL RESEARCH
Published on 01 Jun 2021

METHODS
Published on 21 May 2021
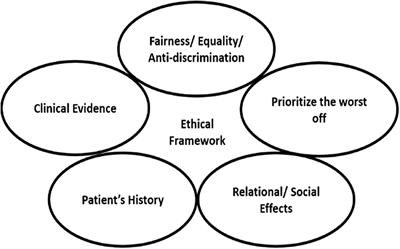
REVIEW
Published on 12 May 2021

ORIGINAL RESEARCH
Published on 07 May 2021
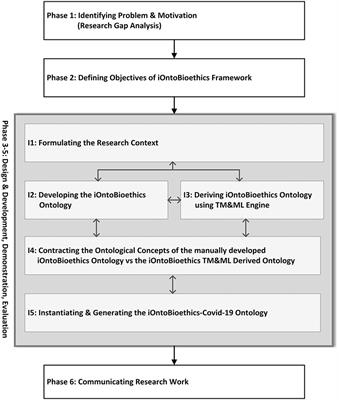
ORIGINAL RESEARCH
Published on 15 Apr 2021

ORIGINAL RESEARCH
Published on 31 Mar 2021
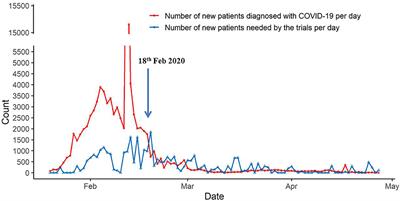
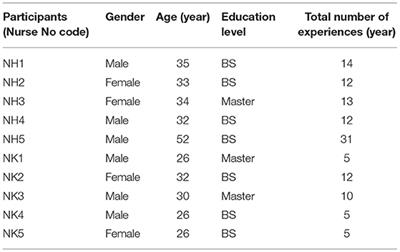
ORIGINAL RESEARCH
Published on 30 Mar 2021

ORIGINAL RESEARCH
Published on 22 Mar 2021