EDITORIAL
Published on 25 Apr 2022
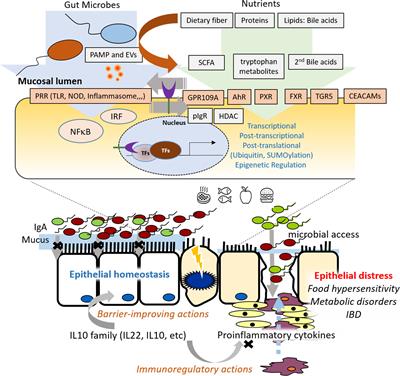
Editorial: Molecular Pathways Controlling Epithelial Inflammation in the Gut
doi 10.3389/fimmu.2022.897587
- 1,257 views
- 1 citation
29k
Total downloads
89k
Total views and downloads
EDITORIAL
Published on 25 Apr 2022

ORIGINAL RESEARCH
Published on 29 Jul 2021

ORIGINAL RESEARCH
Published on 16 Jun 2021

REVIEW
Published on 26 May 2021
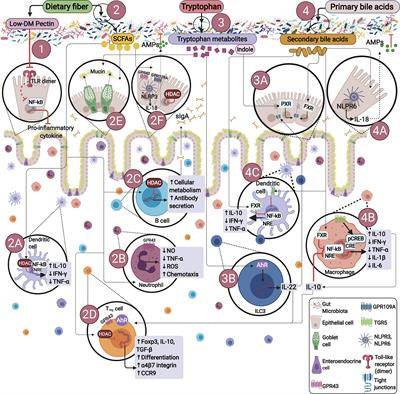
REVIEW
Published on 13 May 2021

ORIGINAL RESEARCH
Published on 30 Apr 2021

MINI REVIEW
Published on 19 Feb 2021

ORIGINAL RESEARCH
Published on 08 Jan 2021

ORIGINAL RESEARCH
Published on 21 Dec 2020
