EDITORIAL
Published on 20 Oct 2021
Editorial: Gene Therapy in the CNS – Progress and Prospects for Novel Therapies
doi 10.3389/fnmol.2021.778134
- 5,091 views
- 4 citations
31k
Total downloads
175k
Total views and downloads
EDITORIAL
Published on 20 Oct 2021
REVIEW
Published on 06 Oct 2021

REVIEW
Published on 14 Jun 2021
ORIGINAL RESEARCH
Published on 21 May 2021
ORIGINAL RESEARCH
Published on 10 May 2021
REVIEW
Published on 11 Mar 2021
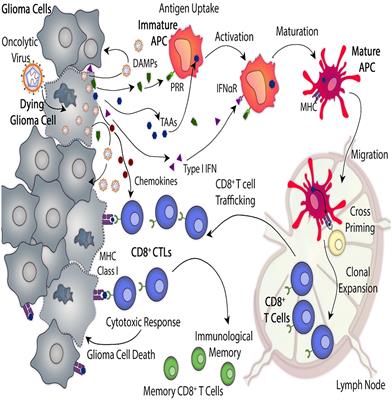
MINI REVIEW
Published on 18 Feb 2021
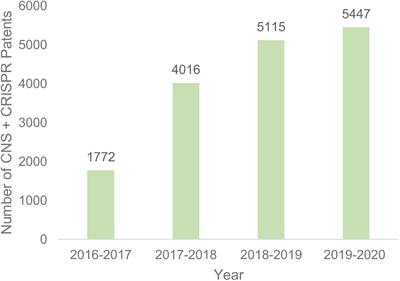
MINI REVIEW
Published on 22 Jan 2021

REVIEW
Published on 11 Jan 2021
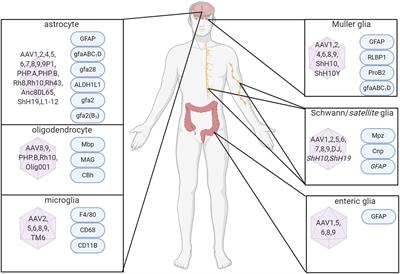
ORIGINAL RESEARCH
Published on 04 Dec 2020
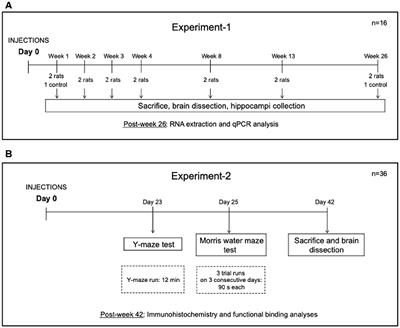
REVIEW
Published on 12 Aug 2020
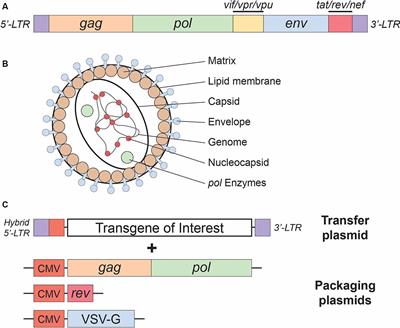
REVIEW
Published on 17 Jul 2020