EDITORIAL
Published on 11 Aug 2021
Editorial: Perinatal Derivatives and the Road to Clinical Translation, Volume I
doi 10.3389/fbioe.2021.741156
- 1,000 views
23k
Total downloads
102k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 11 Aug 2021
MINI REVIEW
Published on 06 Jul 2021
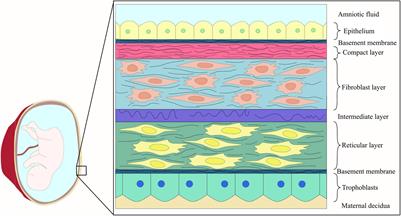
ORIGINAL RESEARCH
Published on 25 Jun 2021
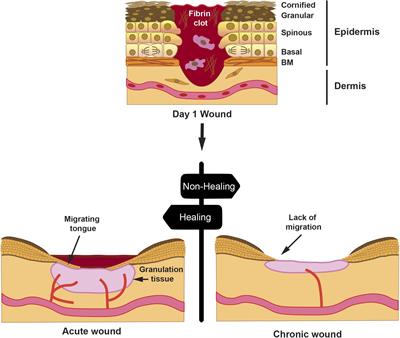
CASE REPORT
Published on 24 Jun 2021
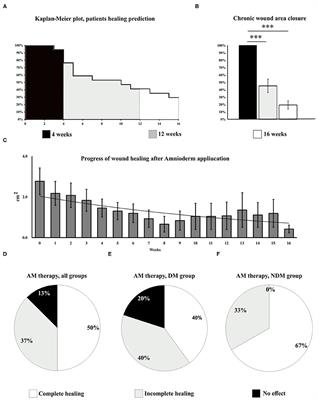
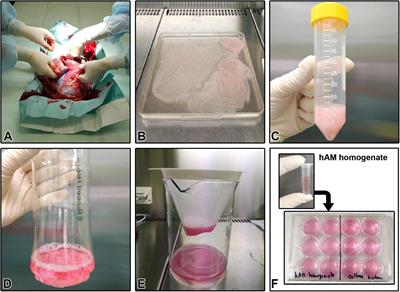
REVIEW
Published on 10 Jun 2021
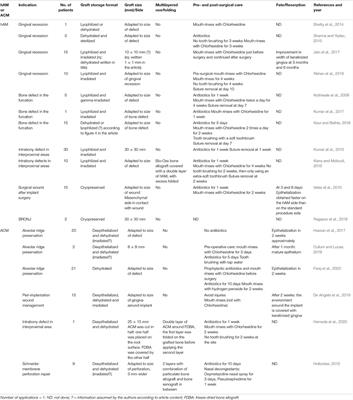
REVIEW
Published on 31 May 2021
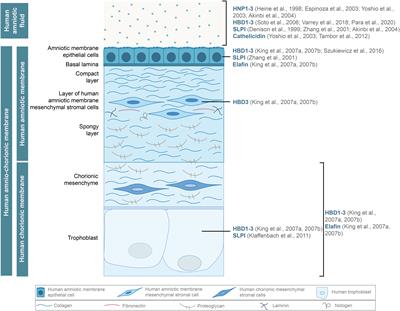
SYSTEMATIC REVIEW
Published on 11 May 2021
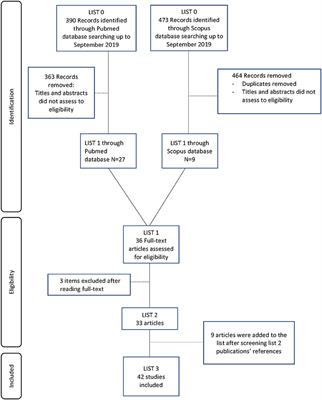
CASE REPORT
Published on 16 Apr 2021
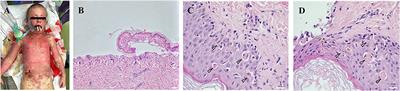
ORIGINAL RESEARCH
Published on 11 Mar 2021
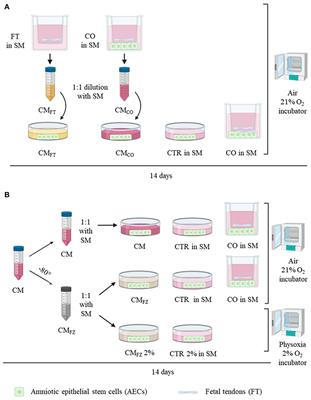
ORIGINAL RESEARCH
Published on 22 Feb 2021
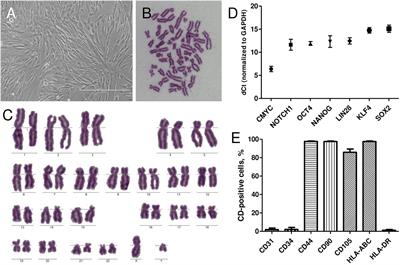
REVIEW
Published on 14 Jan 2021
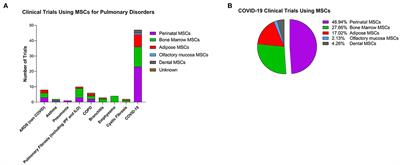
REVIEW
Published on 13 Jan 2021