EDITORIAL
Published on 19 Nov 2021
Editorial: NSAIDs Pharmacogenomics
doi 10.3389/fphar.2021.798447
- 2,294 views
- 2 citations
11k
Total downloads
49k
Total views and downloads
EDITORIAL
Published on 19 Nov 2021
ORIGINAL RESEARCH
Published on 21 Sep 2021
REVIEW
Published on 25 Jun 2021
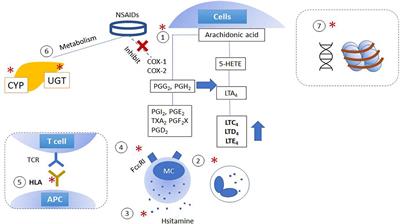
MINI REVIEW
Published on 21 Jun 2021
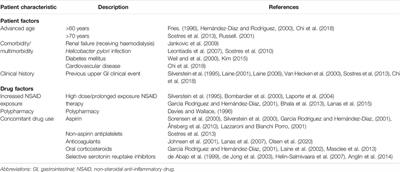
ORIGINAL RESEARCH
Published on 30 Apr 2021

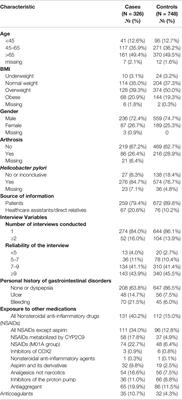
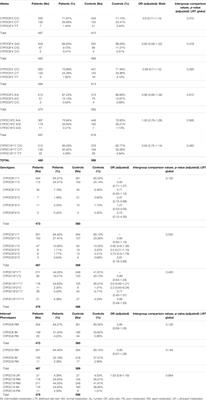
ORIGINAL RESEARCH
Published on 29 Apr 2021
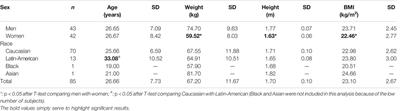
CLINICAL TRIAL
Published on 15 Apr 2021
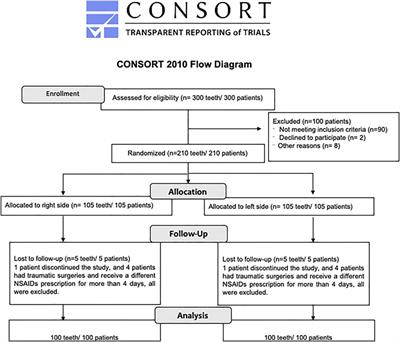
CORRECTION
Published on 17 Jul 2020
ORIGINAL RESEARCH
Published on 09 Jun 2020