EDITORIAL
Published on 28 Oct 2021
Editorial: DNA Vaccines
doi 10.3389/fmedt.2021.782986
- 1,357 views
5,485
Total downloads
49k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 28 Oct 2021
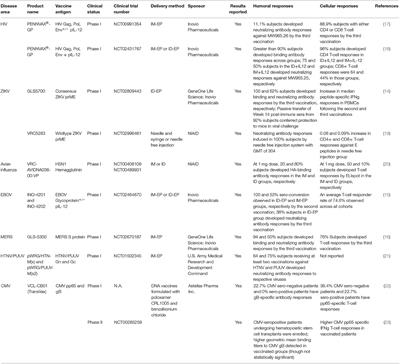
REVIEW
Published on 12 Apr 2021
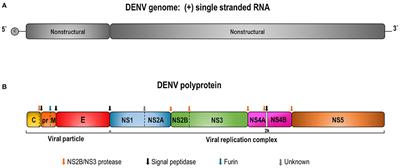
REVIEW
Published on 15 Dec 2020
ORIGINAL RESEARCH
Published on 03 Dec 2020
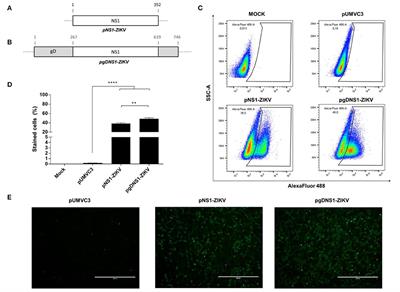
CORRECTION
Published on 27 Nov 2020
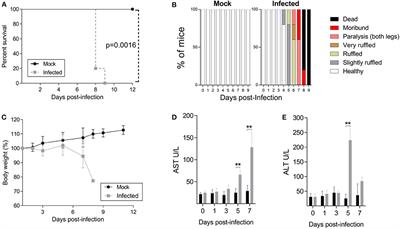
ORIGINAL RESEARCH
Published on 30 Oct 2020
MINI REVIEW
Published on 21 Oct 2020