EDITORIAL
Published on 15 Dec 2021
Editorial: Deprescribing and Minimizing Use of Anticholinergic Medications
doi 10.3389/fphar.2021.820051
- 1,545 views
- 4 citations
6,210
Total downloads
20k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 15 Dec 2021
ORIGINAL RESEARCH
Published on 12 Nov 2021

CASE REPORT
Published on 16 Apr 2021
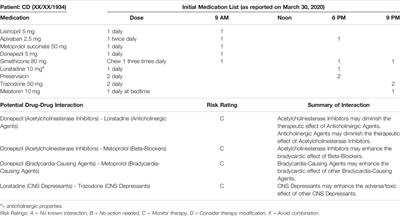
ORIGINAL RESEARCH
Published on 29 Jan 2021

METHODS
Published on 14 Jan 2021

SYSTEMATIC REVIEW
Published on 29 Apr 2020

