EDITORIAL
Published on 20 Jan 2021
Editorial: The Design of Molecular Tools in Relation to Prions and Their Biosafety
doi 10.3389/fbioe.2020.638513
- 1,148 views
- 1 citation
15k
Total downloads
61k
Total views and downloads
You will be redirected to our submission process.
EDITORIAL
Published on 20 Jan 2021
REVIEW
Published on 01 Dec 2020
BRIEF RESEARCH REPORT
Published on 19 Nov 2020
ORIGINAL RESEARCH
Published on 29 Oct 2020
REVIEW
Published on 20 Oct 2020
ORIGINAL RESEARCH
Published on 16 Oct 2020

ORIGINAL RESEARCH
Published on 06 Oct 2020
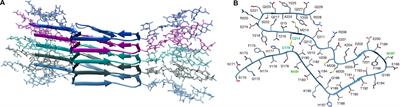
ORIGINAL RESEARCH
Published on 23 Sep 2020
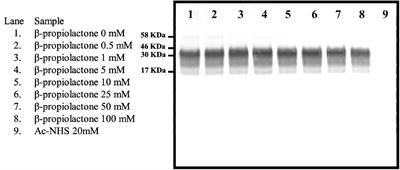
REVIEW
Published on 08 Sep 2020

REVIEW
Published on 27 Aug 2020
CASE REPORT
Published on 06 Aug 2020
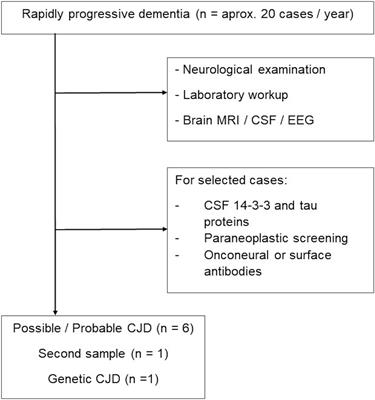
ORIGINAL RESEARCH
Published on 12 Mar 2020

