ORIGINAL RESEARCH
Published on 10 Mar 2020
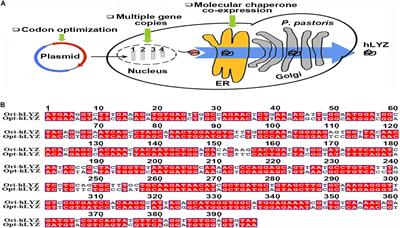
A Combinational Strategy for Effective Heterologous Production of Functional Human Lysozyme in Pichia pastoris
doi 10.3389/fbioe.2020.00118
- 7,224 views
- 24 citations
33k
Total downloads
219k
Total views and downloads
ORIGINAL RESEARCH
Published on 10 Mar 2020

ORIGINAL RESEARCH
Published on 14 Feb 2020

BRIEF RESEARCH REPORT
Published on 29 Jan 2020
ORIGINAL RESEARCH
Published on 17 Jan 2020

ORIGINAL RESEARCH
Published on 10 Jan 2020

REVIEW
Published on 20 Dec 2019