- 1Movement Referrals: Independent Veterinary Specialists, Runcorn, United Kingdom
- 2Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Leahurst Campus, Neston, United Kingdom
- 3Translational Research in Pain Program, North Carolina State University, Raleigh, NC, United States
- 4Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, United States
- 5The Sands Veterinary Practice, Apex House, Chester, United Kingdom
- 6The Market Town Vet, Oswestry, United Kingdom
- 7Woolton Veterinary Centre, Liverpool, United Kingdom
- 8Vetcare Ltd., Leigh, United Kingdom
- 9Wigan Vets for Pets, Wigan, United Kingdom
- 10The Village Veterinary Surgery, Formby, United Kingdom
- 11Hollybank Veterinary Centre Ltd., Sandiway, United Kingdom
Bedinvetmab (Librela®), a fully canine anti-nerve growth factor monoclonal antibody, was compared to the non-steroidal anti-inflammatory drug (NSAID) meloxicam in dogs for the management of osteoarthritis-related pain in a randomised, open-label, multicentre, parallel-group study. Subjects were recruited from general practices as client-owned dogs with appendicular osteoarthritis. Dogs were block randomised 1:1 to either daily oral meloxicam or bedinvetmab, administered subcutaneously once a month. The primary endpoint for efficacy was the change from baseline in the Canine Orthopaedic Index (COI) score. Linear mixed-effects models were used for statistical analysis conducted on a per-protocol and intent-to-treat basis. We hypothesised that bedinvetmab would demonstrate superior efficacy and safety compared to meloxicam; the number needed to harm (NNH) for meloxicam, relative to bedinvetmab, was calculated. Of the 190 screened dogs, 101 were randomised (bedinvetmab 52; meloxicam 49). Overall, both treatment groups showed a significant reduction in COI scores relative to baseline (p < 0.001). The bedinvetmab group experienced a larger mean reduction in COI scores, but this was not statistically significant. A significant effect of the visit was observed, with later visits showing a significantly greater reduction in COI compared to Visit 2 (p < 0.001). The bedinvetmab group reported four (AEs), whilst the meloxicam group reported 17, with nine of those being gastrointestinal system disorders. Additionally, more dogs in the bedinvetmab group completed the study (n = 44) compared to those in the meloxicam group (n = 33). This is the first study to compare bedinvetmab to an NSAID for the management of osteoarthritis-related pain in dogs. The results suggest that both products are equally effective in managing OA pain, with efficacy improving over time for both treatments. Bedinvetmab was associated with fewer AEs. These data will aid clinicians and pet owners in choosing analgesic options for dogs with osteoarthritis.
Introduction
Osteoarthritis (OA) is the most common orthopaedic disorder in dogs. Prevalence estimates vary depending on the case definition; one retrospective study of practice management system data from multiple primary care practices in the United Kingdom reported an annual period prevalence of 2.5% (1). However, a prospective survey of dogs aged 8 months to 4 years, using radiographic and clinical case definitions, found that 39.8% developed radiographic OA, and 23.6% developed clinical OA (2). Furthermore, in that study, only 2 of 29 dogs identified with clinical OA were receiving pain management. In another study, when dog owners were surveyed with an OA checklist and responded positively to at least one item (n = 550), 38% of their dogs were confirmed to have OA after a veterinary examination (3). Such data indicate that OA is not only a very common condition but also that there is a significant unmet need for education for owners and pain management for dogs suffering from this condition.
The majority of dogs with osteoarthritis are managed using conservative and medical approaches. Since the 1990s, non-steroidal anti-inflammatory drugs (NSAIDs) have been approved for dogs to treat pain associated with osteoarthritis. Numerous studies document the effectiveness of NSAIDs in dogs (4–9), along with research on the adverse events (AEs) that may occur with their use (10, 11).
Meloxicam is a commonly used NSAID that was licensed in the 1990s for the treatment of canine osteoarthritis (OA) (9) and is used in many territories globally. Previous studies have reported the efficacy of meloxicam and also reported a gastrointestinal adverse event rate of 12% (12) and 15% (5).
In 2021, a novel class of analgesics was licensed in Europe and the United Kingdom for relieving pain associated with OA. Bedinvetmab (Librela®, Zoetis) is a fully canine monoclonal antibody that targets nerve growth factor (NGF) and has since been licensed in over 50 territories, including the United States, Canada, Latin America, Asia, and Australia. Nerve growth factor was identified as a key mediator of joint pain in preclinical animal models of OA (13) and is a neurotrophic factor involved in pain signal transduction and nociceptor receptor gene expression, playing an important role in pain signalling in OA. Nerve growth factor is a soluble protein released by mast cells (14), macrophages (15), and chondrocytes (14). It binds to the high-affinity NGF-specific tropomyosin receptor kinase A (TrkA) on peripheral nociceptors, potentially leading to both peripheral and central sensitisation.
Furthermore, when NGF binds to TrkA receptors on immune cells, it triggers the release of additional pro-inflammatory mediators, including NGF itself (16). In the periphery, NGF also binds to TrkA receptors on mast cells and other immune cells, resulting in the release of inflammatory mediators such as histamine, serotonin, and NGF itself. Thus, NGF can trigger peripheral sensitisation and sensitise adjacent nociceptive neurones through the release of these inflammatory mediators (17–20). The nerve growth factor also drives neoinnervation in the synovium and subchondral bone (14). Blocking NGF binding to TrkA has been demonstrated to reduce OA pain in preclinical models (13), and subsequent clinical trials have confirmed the efficacy of anti-NGF antibodies in humans (21), cats (22), and dogs (23–25) with OA. In addition, using a validated Health-Related Quality of Life instrument, a clinical study conducted in the United Kingdom reported that bedinvetmab improved the quality of life in dogs across multiple domains (26).
The European and American field trials of bedinvetmab compared dogs with osteoarthritis receiving bedinvetmab to those receiving a placebo (24, 25). Whilst these studies demonstrated efficacy for regulatory purposes, clinicians are also interested in the efficacy and safety of new medicines when compared to existing ones.
Three published pain guidelines [AAHA (27), WSAVA (28), COAST (29)] recommend both NSAIDs and anti-NGF monoclonal antibodies as appropriate first-line treatments for dogs with OA pain. Furthermore, bedinvetmab was recommended (by majority consensus) to manage OA pain in dogs with moderate OA for a minimum of 2 months (COAST).
Accordingly, the study reported herein was designed to compare the efficacy and safety of bedinvetmab with that of the NSAID meloxicam in a randomised, open-label, parallel-group clinical trial involving dogs with OA over a 56-day treatment period. The authors hypothesised that bedinvetmab would exhibit superior efficacy and safety compared to meloxicam.
Materials and methods
Study design
This randomised, open-label, multicentre clinical comparator study was conducted following an ethical review by the Zoetis VMRD ethical review assessment committee (Study 2INTORCADPAIN01).
Eligible dogs participating through veterinary clinics were randomly assigned to one of two treatment groups based on the order in which they presented to the clinic. The plan was to have at least 46 evaluable cases in each treatment group, and no single clinic was to enrol more than 40% of the total number of evaluable cases. Each participating site was to attempt to enrol at least two complete blocks (four animals).
Sample size estimates were determined based on the assumption that bedinvetmab (Librela®, Zoetis) would be superior to meloxicam in terms of efficacy. The primary outcome measure was the Canine Orthopaedic Index (COI), a validated client-reported outcome measure (CROM) (30–32). Sample size estimates using the COI required several assumptions: first, published work indicated a standard deviation for COI in a population of dogs with OA to be “10” for the aggregate COI score (32) and that treatment with the NSAID carprofen resulted in a mean decrease in COI score of “12.”
In the absence of published data, it appeared likely that meloxicam would have similar efficacy to carprofen in terms of impact on the COI score (8). Similarly, without published data suggesting otherwise, we set an arbitrary clinically meaningful difference between meloxicam and bedinvetmab at 6 points on the COI. Considering a 10% dropout rate and targeting 80% power indicated that we would need to recruit group sizes of 46.
Study population
Client-owned dogs were recruited from eight primary care veterinary practices in the United Kingdom between March 2023 and March 2024. The recruited cases included dogs with incident OA or those with previously diagnosed OA who were receiving only nutraceuticals and were considered eligible for screening. Practice raised awareness of the study amongst their existing client base using provided resources, including in-clinic posters, websites, and social media posts. Clients could withdraw their dogs from the study for any reason at any time, and whenever possible, the reason for withdrawal was recorded.
Inclusion criteria
Clinical and radiographic evidence of OA in dogs aged 1 year or older was confirmed in at least one major joint of either a thoracic (shoulder, elbow, carpus) or pelvic (hip, stifle, tarsus) limb during a screening visit. The dog was expected to benefit from continued treatment for a duration of 2 months for clinical signs of OA. The severity of at least one veterinary categorical assessment (general musculoskeletal condition, pain on palpation/manipulation of joint(s), lameness/weight bearing) was at least “moderate” (scale: clinically normal, mild, moderate, severe, nearly incapacitating) at both the screening and day 0. The COI, as assessed by the owner, was 26 or higher (maximum score 64). Veterinarians confirmed that the dogs were generally in good health, concomitant diseases were well controlled, and clinical pathology results (blood and urine) were satisfactory.
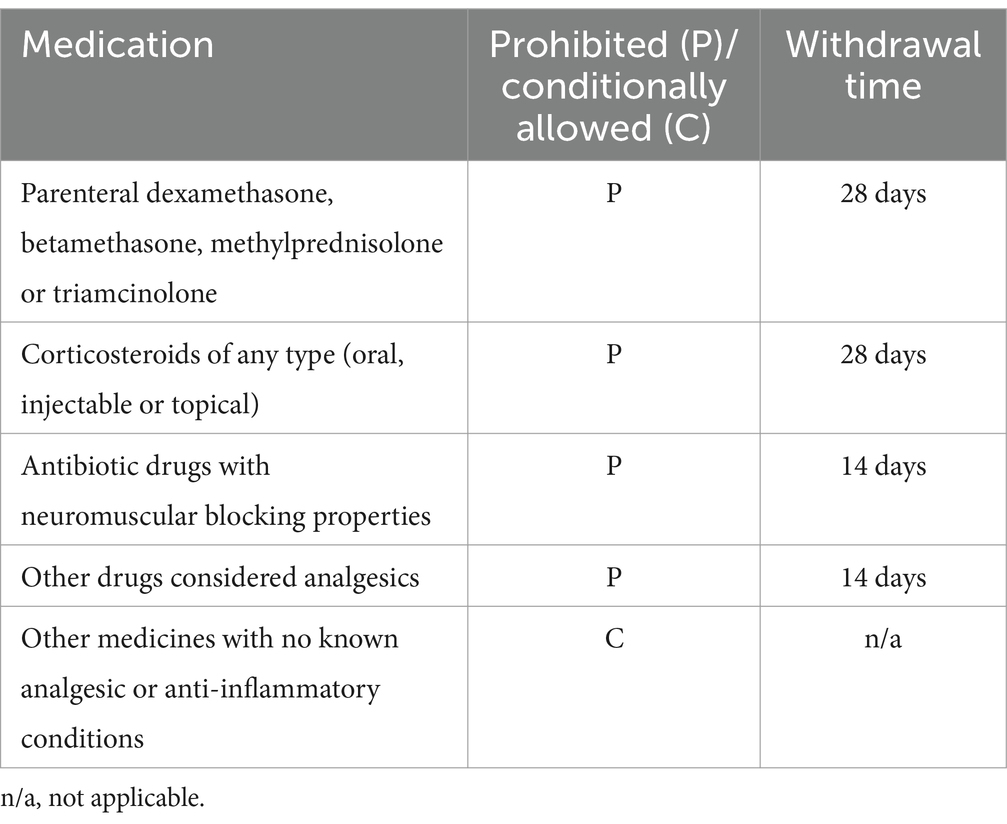
Specifically, the packed cell volume and total protein levels were within the normal range, with blood urea nitrogen (BUN) and creatinine also either within the normal range or a maximum of 10% above it. Alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels were either within the normal range or a maximum of three times the normal range. If ALT or ALP exceeded twice the upper limit of the normal range, bile acids were tested; if the bile acids were normal, the subject was included. If a dog was receiving any concomitant medications listed in Table 1, both the owner and the examining veterinarian had to agree to adhere to all withdrawal times, minimal usage, and frequency of usage. If a dog was receiving a conditionally allowed medication (Table 1), the dosage and dosage rate were not expected to change during the study. Dogs receiving nutraceuticals for OA [e.g., “joint” diets, glucosamine/chondroitin sulphate, green-lipped mussel extract, methylsulfonylmethane (MSM), fish oil, avocado soybean unsaponifiable (ASU)] were to continue at the same dosage throughout the study. The dog’s owner had to commit to evaluating their dog over a continuous period of 8 weeks. Additionally, the owner needed to anticipate a stable lifestyle (e.g., no impending major life events or periods of absence). The owner also had to agree to administer the medication according to instructions if randomised to the meloxicam group and to maintain the dog’s basic management (exercise, routines) throughout the 8-week period.
Exclusion criteria
The dogs excluded from the study included those enrolled in a clinical trial ≤30 days before day 0 or involving an injectable product <90 days before day 0; those intended for breeding, lactating, or pregnant; and those with lameness due to primary immunologic, neurologic, infectious, or neoplastic conditions. Additionally, dogs with ligament ruptures in the last 12 months or non-healed fractures were excluded. Dogs with a history of injury leading to neurologic deficits or intervertebral disc disease (if expected to interfere with efficacy assessment) were also excluded, as were those administered prohibited medication (see Table 1). The use of conditionally allowed medication was allowed under specific conditions regarding minimal use and frequency (see Table 1). However, dogs receiving any of the prohibited medications listed in Table 1 were excluded unless they had completed the respective withdrawal times. Further exclusions included dogs with conditions likely to require surgical intervention during the study; those whose lameness was known to be associated with neoplasia, primary neurologic or immunologic disorders (e.g., immune-mediated polyarthritis), infections (e.g., septic joint), recent joint trauma, or non-healed fractures. Dogs that had started a physical therapy programme less than 8 weeks before screening or a weight loss programme less than 8 weeks before screening (unless on a stable weight loss regimen) were also excluded. Finally, dogs with known hypersensitivity to the active substances or any excipients were ineligible for enrolment.
Randomisation
Enrolled dogs were randomised in a 1:1 ratio to receive either bedinvetmab or meloxicam, using a randomised complete block design with a one-way treatment structure that was replicated across multiple clinics. All data collection and randomisation for the study were conducted using the Castor electronic data collection software platform (Castor EDC, Amsterdam, Netherlands).
Dogs were randomly assigned to groups based on their order of entry into the clinic study and the randomisation provided by the Castor platform. Within each site, blocks of two dogs were created according to the order of enrolment. In each block, dogs were randomly allocated to either meloxicam or bedinvetmab. Day 0 was the day a dog received its first dose.
Treatment administration
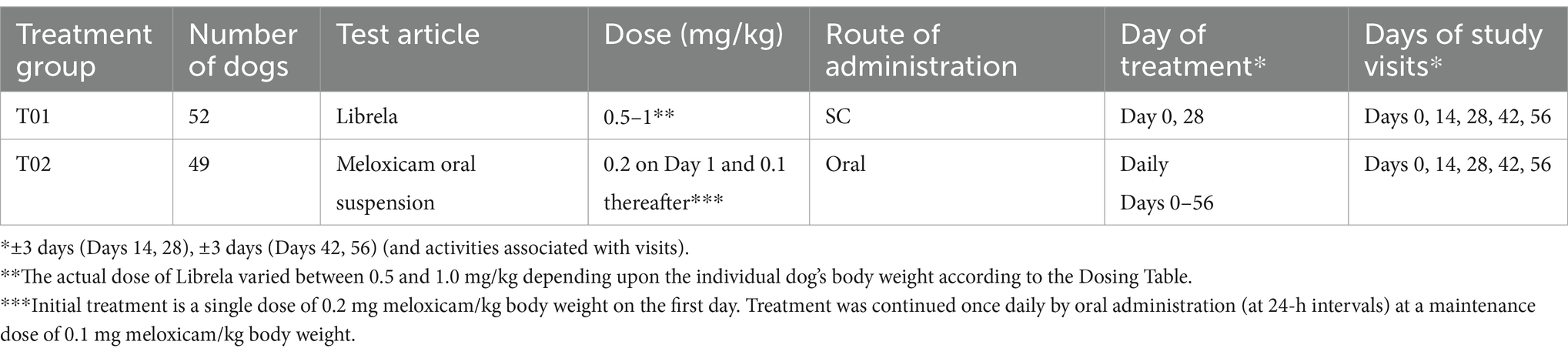
For dogs randomised to bedinvetmab, the UK dosage chart (Supplementary material) ensured a bedinvetmab dosage of 0.5 to 1.0 mg/kg using a ready-to-use formulation in single-use 1 mL vials (vial strengths: 5, 10, 15, 20, or 30 mg/mL). The use of the dose chart results in a minimum target dose of bedinvetmab of 0.5 mg/kg (0.23 mg/lb). Both doses of bedinvetmab (day 1 and day 28) were administered by the attending veterinarian. Dogs randomised to meloxicam were administered an initial dose of 0.2 mg/kg by subcutaneous injection on day 1 from the veterinarian, and an oral meloxicam suspension was dispensed for administration at 0.1 mg/kg daily for the remainder of the study period (an additional 55 days) by the owner.
Study schedule
A screening visit took place 0–21 days before day 1. The screening included the completion of the COI by the owner, a veterinary categorical assessment by the veterinarian, haematology, blood biochemistry, urinalysis, and a radiographic examination to confirm the presence of OA. Baseline COI data collection took place on day 1, prior to enrolment. Four additional clinic visits followed day 1, specifically on days 14, 28, 42, and 56. Dogs receiving bedinvetmab were dosed by the veterinarian on days 1 and 28. A veterinary physical examination was conducted at each visit, during which veterinarians completed the veterinary categorical assessment and documented any AEs or medications, if applicable. Within 3 days of each visit, owners, blinded to their prior assessments, completed the COI electronically via the Castor EDC platform. At the final clinic visit (day 56 ± 3 days), in accordance with recommended best practice for dogs treated with NSAIDs (33), particularly those on meloxicam, blood was collected for haematology and serum biochemistry, and urine was collected for urine specific gravity.
Escape clause and the use of rescue and prohibited therapies
A dog could be withdrawn from the study by the veterinarian or owner at any time. Prohibited or conditionally allowed medications could be administered prior to withdrawal, if necessary, for animal welfare reasons. “Rescue” treatment for an OA-related issue (e.g., lack of efficacy) was considered a prohibited treatment. A “prohibited” treatment that was not classified as rescue treatment was one that could interfere with efficacy assessments and was used for reasons unrelated to OA.
Efficacy outcome measures
The primary measure of efficacy was the change from the baseline COI score. Dogs with protocol deviations affecting integrity or data collection were excluded from the efficacy analyses. After enrolment, dogs were withdrawn from the study for using prohibited treatments. Dogs withdrawn for loss of efficacy or rescue treatment were included in the intent-to-treat analyses.
Safety measures
For safety, AEs were recorded and categorised according to the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA) (34). For dogs that received meloxicam, clinical pathology data on day 56 were compared to those collected on day 1.
Compliance
Compliance with the administration of bedinvetmab was ensured by accurately recording a successful subcutaneous injection of the correct vial size on the case report form (CRF) within the Castor EDC platform. Similarly, compliance with administering the loading dose of meloxicam and dispensing the correct dose and bottle size of meloxicam was recorded in a similar manner. Additionally, the owner’s compliance in dosing meloxicam at home was evaluated by weighing the bottle of meloxicam during each visit.
Statistical analysis
Data analysis was conducted using R (version 4.2, R Foundation for Statistical Computing) and MLwiN (version 3.10, Centre for Multilevel Modelling, University of Bristol) on a “per protocol” and “intent to treat” basis. For the intent-to-treat analysis, missing data were imputed using multiple imputations by chained equations with the R package mice. The COI data were analysed separately using a general linear mixed effects model for repeated measures, with the change in COI from baseline as the outcome. The initial COI score served as a covariate, incorporating random effects of site and dog (for repeated measures) and fixed effects of treatment and visit.
Results
Demographic data
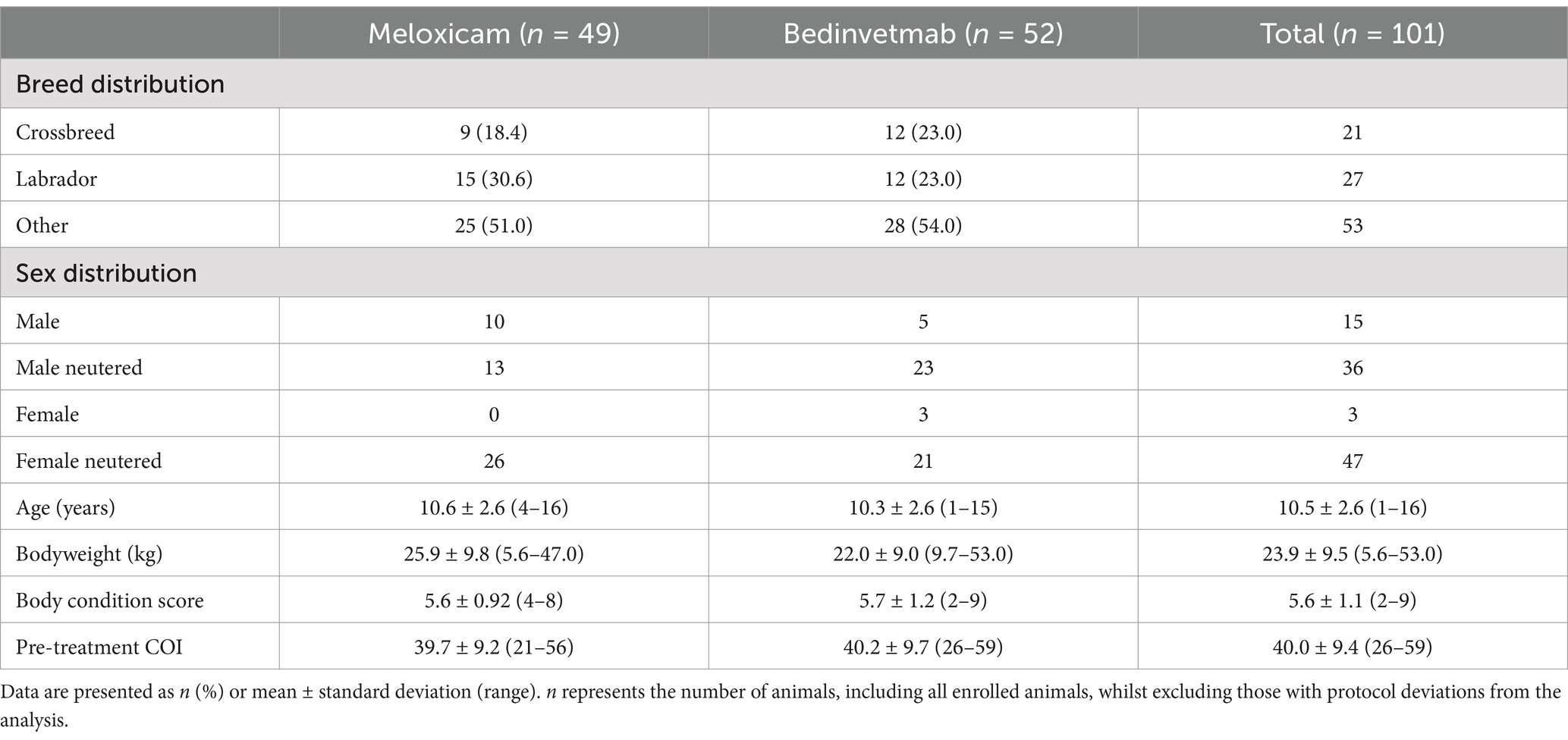
Eligibility criteria were not met for 89 of the 190 dogs screened; consequently, 101 dogs (meloxicam, n = 49; bedinvetmab, n = 52) were randomised (Tables 2, 3; Figure 1). A total of 25 dogs were excluded from this study because their COI scores were below 26 at the screening visit. Two dogs were mistakenly included in randomisation as their initial visit COI was less than 26; these dogs were not considered in any further analyses (including the intent-to-treat analysis), resulting in a total of 99 dogs available for analysis. The Labrador Retriever was the predominant breed (n = 27; 27%), followed by the Cocker Spaniel (n = 6; 6%) and the Border Collie (n = 4; 4%). No other individual pure breeds comprised ≥3% of the total, and 21 dogs (21%) were crossbreeds. The most common index joints in the study population were the hip (n = 41; bedinvetmab, n = 22; meloxicam, n = 19), followed by the elbow (n = 33; bedinvetmab, n = 17; meloxicam, n = 16). The remainder (n = 25) had OA of the stifle, shoulder, or tarsus.
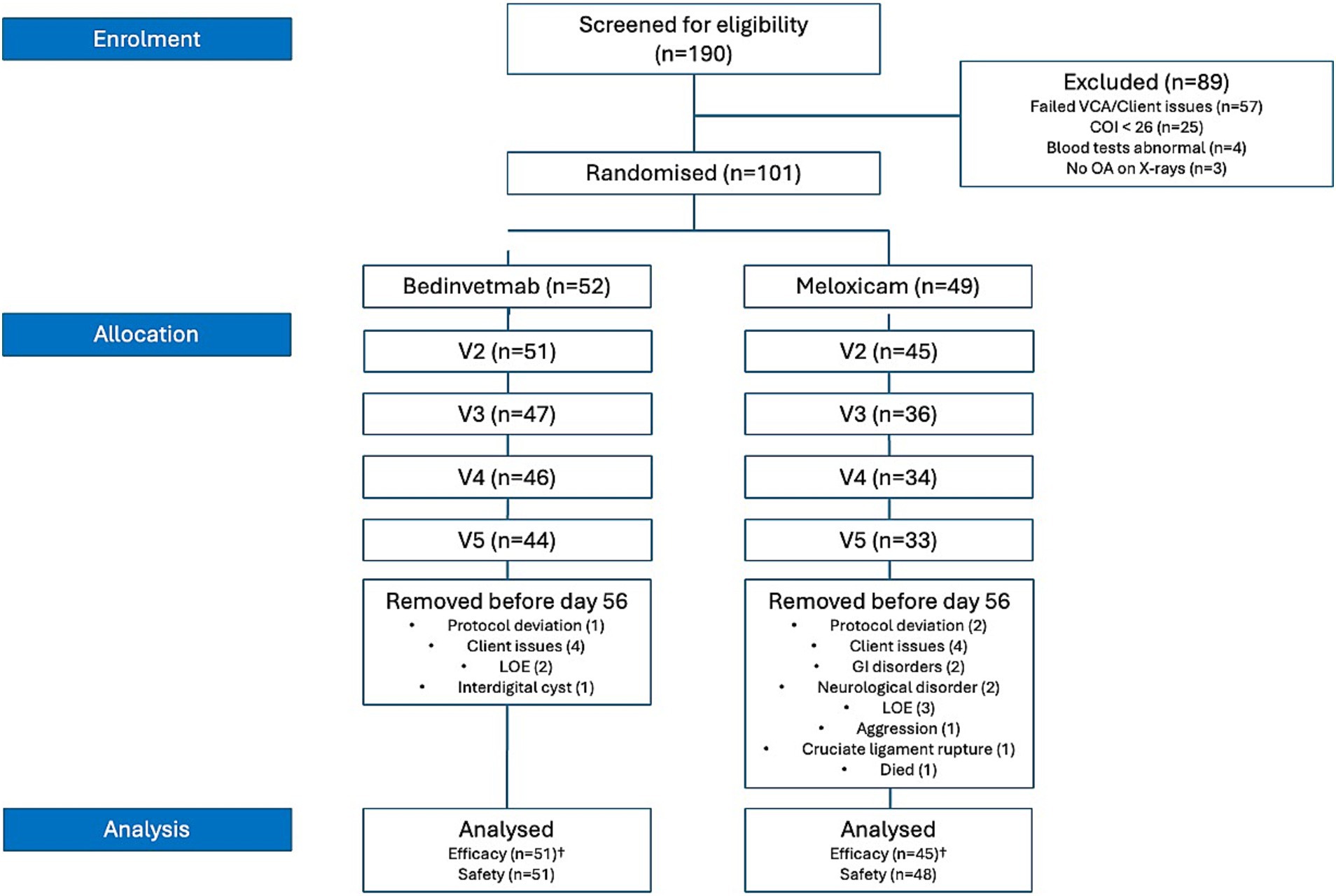
Figure 1. CONSORT flow diagram depicting all cases recruited for the 2-month study. n, number; COI, Canine Orthopaedic Index; VCA, Veterinary Categorical Assessment; LOE, lack of efficacy. †Data excluded due to protocol deviations (i.e., eligibility criteria not met, owner did not complete the COI assessment, animal withdrawn for developing an unrelated medical or surgical condition, owner non-compliance/lost to follow-up, or administration of prohibited treatments) clarifies the cases excluded from the efficacy analysis. All animals were included in the safety data analysis.
Efficacy assessment
Of the enrolled dogs, 23 were withdrawn prior to visit 5 (day 56) (meloxicam, n = 15; bedinvetmab, n = 8) (Table 4). Five dogs were withdrawn due to loss of efficacy (meloxicam, n = 3; bedinvetmab, n = 2). Six animals were withdrawn for medical or surgical conditions deemed unrelated to the test article (meloxicam, n = 5; bedinvetmab, n = 1), and six were withdrawn due to owner non-compliance or because they were lost to follow-up. Data were excluded for protocol deviations related to visits outside the permitted window (meloxicam, n = 1). No dogs were withdrawn due to the administration of prohibited medication.
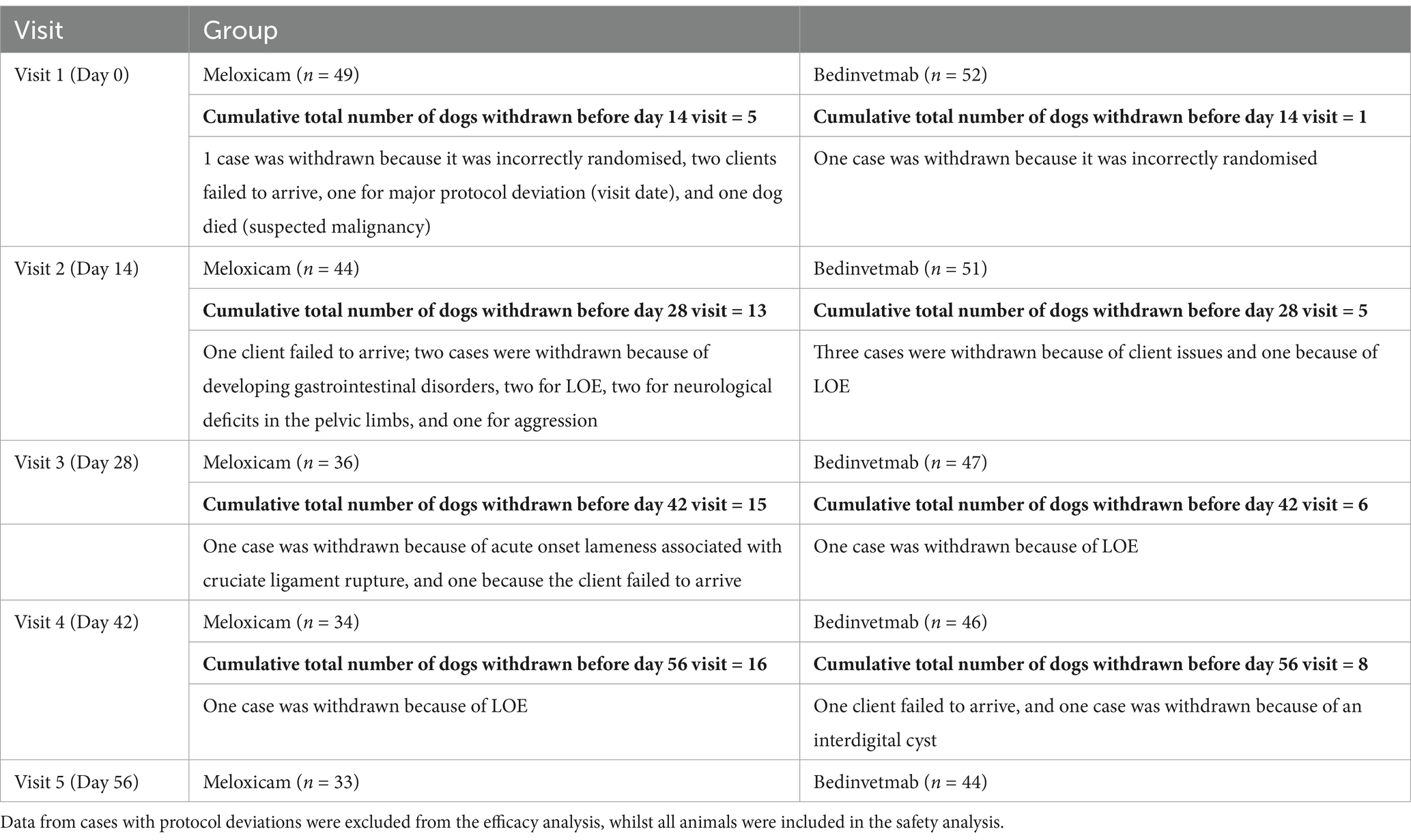
Table 4. Overview of dogs with osteoarthritis that were administered either daily oral meloxicam or monthly subcutaneous injections of bedinvetmab for 2 months and were withdrawn from the study before the day 56 visit.
Owner assessments (COI)
Per protocol analysis
For the change in COI from baseline, there was no significant effect of treatment (i.e., meloxicam vs. bedinvetmab), with p = 0.57 (Figure 2). There was a significant effect of visit, with all later visits showing a significantly greater reduction in COI compared to Day 14 (p < 0.001), indicating that clinical status in both groups improved significantly, as reflected by owner COI scores.
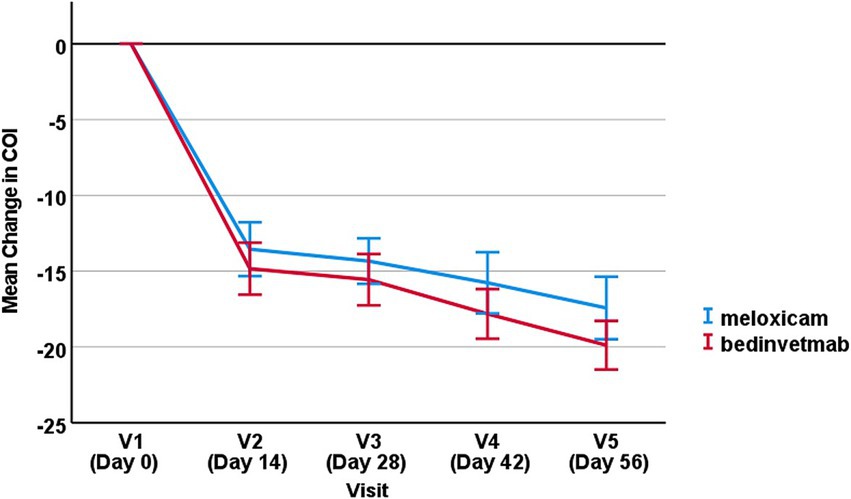
Figure 2. Mean change in COI score from baseline (±SEM) for visits 2–5 for dogs treated with bedinvetmab and those treated with meloxicam.
Intent-to-treat analysis
For the change in COI from baseline, there was no significant effect of treatment (i.e., meloxicam vs. bedinvetmab) with p = 0.42. However, a significant effect of visit was observed, with Days 42 and 56 showing a significantly greater reduction in COI compared to Day 14 (p < 0.001).
Safety assessment
AEs and concomitant medications
Three AEs were associated with clients (two incidents related to clients’ health and one involving a client who lost their means of transport); these AEs were excluded from further analysis.
Per protocol analysis
At least one AE was reported for 17 dogs in the meloxicam group and four dogs in the bedinvetmab group (see Tables 5, 6). The most commonly reported AEs were gastrointestinal disorders, including diarrhoea, and emesis, which occurred in the meloxicam group. Based on these AE rates, the number needed to harm for meloxicam compared to bedinvetmab was 5 (95% Confidence Interval 2.8–27.2). Two dogs in the meloxicam group were euthanised: one between visits 1 and 2 due to suspected malignancy (not confirmed) and the other between visits 2 and 3 due to aggression that resulted in a bite injury to a person.
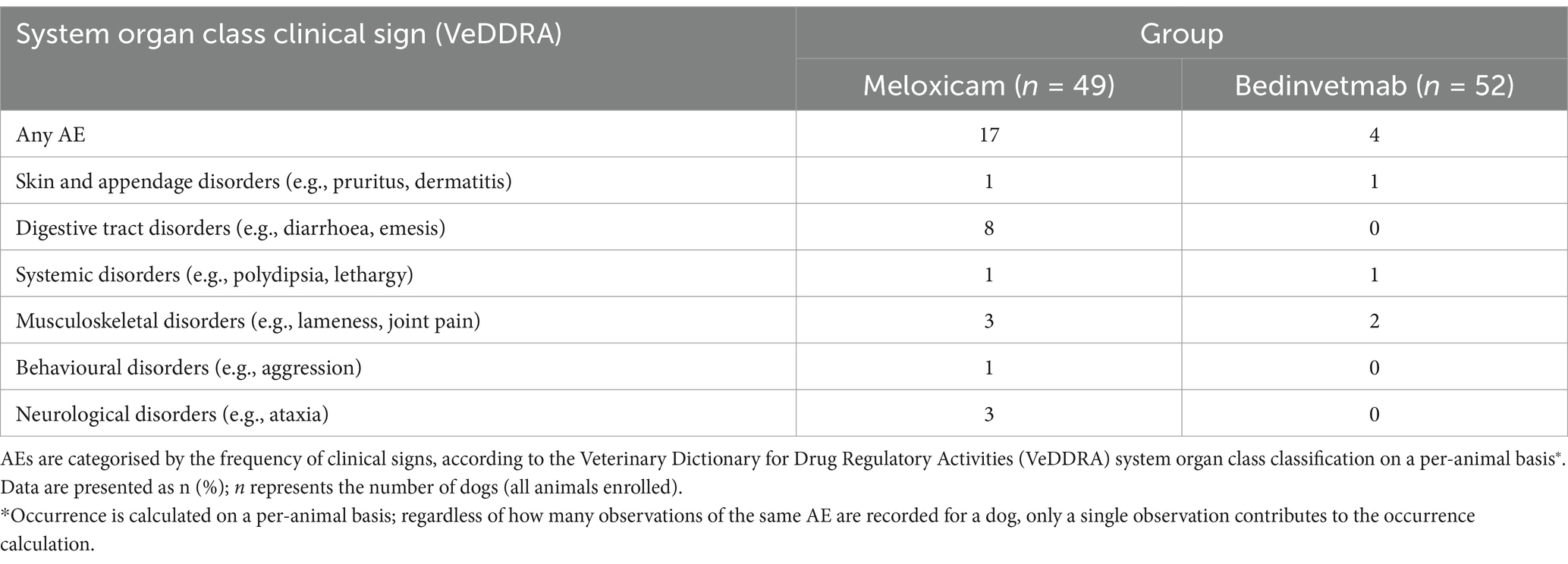
Table 5. AEs in dogs with osteoarthritis receiving daily doses of meloxicam or monthly subcutaneous injections of bedinvetmab (the bedinvetmab group) occurring at least once.
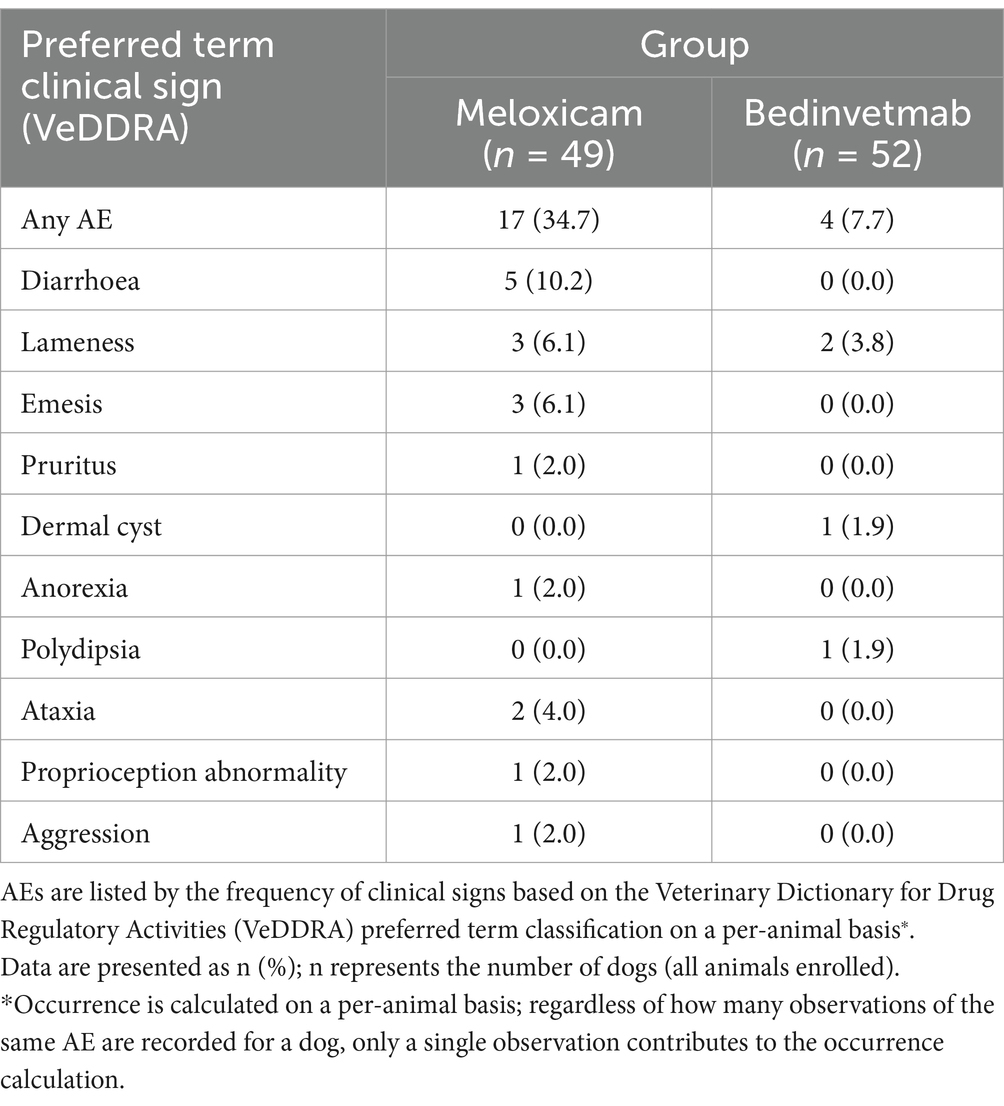
Table 6. AEs occurring at least once in dogs with osteoarthritis receiving daily doses of meloxicam or monthly subcutaneous injections of bedinvetmab.
Clinical pathology
At screening, the mean and median values for all haematology and serum chemistry analytes in both groups fell within the reference ranges for the measured analytes in 98 of the 101 enrolled dogs. In three dogs, ALT and/or ALP levels were elevated during the screening visit, but bile acids remained within normal limits, and the dogs were enrolled according to the protocol.
Between days 1 and 56, changes in clinical pathology results (either increases or decreases compared to pre-treatment) were observed in the meloxicam group; however, only the changes in creatinine were statistically significant. Creatinine concentrations increased significantly from day 1 to day 56 (p = 0.031). No significant changes were noted in BUN (p = 0.32) or urine-specific gravity (p = 0.27). Liver enzymes, including ALT and ALP, did not exhibit significant changes from day 1 to day 56 (p = 0.34 and p = 0.10, respectively). No other significant changes in the measured parameters were observed.
Compliance
Compliance for bedinvetmab was recorded at 100% because all investigators administered the subcutaneous injection in the clinic and documented it in the EDC system. For meloxicam, analysing the data proved challenging. We requested that the meloxicam bottle be weighed at each visit, as this would allow us to estimate compliance by recording the weight loss between visits and the product’s specific gravity. Data indicated that five different brands of meloxicam were used in the study: Metacam (Boehringer Ingelheim), Loxicom (Norbrook Laboratories Ltd.), Rheumocam (Chanelle Pharma), Meloxidyl (Ceva Animal Health Ltd.), and Inflacam (Virbac Ltd.). Whilst the specific gravity for Metacam is published (1.56), specific gravity values for other brands were not always available, and we were advised that these values could vary from batch to batch. The best analysis we could perform suggested an average underdosing of meloxicam by 32%, but the confidence in this result cannot be considered high.
Discussion
The canine population in this study was similar to that in other studies examining the efficacy of meloxicam (9, 35) and bedinvetmab (24, 25) in dogs with OA pain. Dogs identified with incident OA in primary care settings tend to be older, as demonstrated in this study, with a mean age exceeding 10 years. We excluded dogs younger than 1 year because bedinvetmab is not approved for this age group. The most prevalent breed in this study was the Labrador Retriever, a popular breed known for its predisposition to OA in the elbow and hip joints (36–38). The most common index joints in the studied dogs were the hip joint, followed by the elbow joint, showing a similar distribution between both groups.
Although no published data exist on the relationship between COI scores and the severity of OA as assessed by other measures, the dogs in this study were required to have a minimum baseline score of 26 or higher. Additionally, one category in the Veterinary Categorical Assessment needed to be rated as at least moderate, and based on that assessment, the dogs in this study should be considered to have moderate or more severe osteoarthritis.
Outcome measures for canine OA have evolved over time, with validated CROMs being used more frequently. In recent years, regulatory studies in canine OA have used the Canine Brief Pain Inventory (CBPI) (24, 25, 39) as a primary outcome measure because this instrument has established thresholds for defining responders and non-responders (40). However, a recent COSMIN-based review concluded that two other CROMs, namely the COI and the Liverpool Osteoarthritis in Dogs (LOAD), also provided sufficient evidence for their use in evaluating dogs with osteoarthritis (41).
“Minimal clinically important differences” (MCIDs) have recently been published for COI and LOAD (42, 43). In veterinary medicine, the MCID represents the smallest improvement considered valuable by a client. The availability of MCID estimates has facilitated the use of these instruments in clinical studies, such as the one reported herein, and should now enable their use in regulatory studies. Since the CBPI is designed to provide mean scores for multiple items in the “pain severity score” and “pain interference score” categories, it may be less responsive to changes in clinical status compared to tools like COI and LOAD, which are aggregate scores of several items. Some evidence in the published literature supports this idea (23).
The MCID for COI has been estimated at 14 (42, 43). Because we wanted to detect a reduction in COI scores equal to or greater than the MCID and to assess whether one treatment was superior to the other, we established an inclusion criterion of a minimum COI score of 26 at day 0. Data on the use of COI in clinical research remain relatively limited (43–53), as this instrument has only been validated in recent years (30–32), whereas the CBPI and LOAD scales have been validated for considerably longer periods (54–57). Nevertheless, the results of our study suggest that this threshold of 26 allowed us to detect a significant reduction in COI scores. However, it has also led to attrition during the screening process, with 25 dogs being excluded due to COI scores below 26.
In the absence of published information, for sample size estimates in this study, we established a threshold of a 6-point difference in COI scores to indicate a clinically meaningful difference between bedinvetmab and meloxicam. A post-hoc analysis of another dataset available to us (43) supported this decision. In that previous study, changes in COI scores were categorised based on client responses to an anchor question, with the average difference between the “somewhat better” and “much better” groups being 4.5 points on the COI (rounded up 5 points) (43).
The primary outcome measure in this study was defined as the “change in COI score from baseline.” Both treatments resulted in a statistically significant reduction in COI scores over time, and bedinvetmab performed equally well to meloxicam, with a non-significant difference in efficacy between treatments.
Although the difference was non-significant at all timepoints, the mean reduction in COI scores was greater in the bedinvetmab group than in the meloxicam group at each sampling point.
On day 14 (visit 2), on average, the dogs in the bedinvetmab group experienced a reduction in COI scores of 14 points, which is equal to the MCID. In contrast, the dogs in the meloxicam group did not see a decrease in COI scores that equalled or exceeded the MCID (the mean change in COI score for meloxicam was 12.9 points). Throughout the study, both groups experienced further reductions in mean change from baseline COI scores, with the total mean reduction for bedinvetmab being 19.7 points and for meloxicam being 17.1 points. These data support the use of analgesics for managing OA pain for a duration sufficient to observe maximum benefits for affected dogs. The phenomenon of increasing benefits from continuous analgesia has been previously reported (4, 5), and expert guidelines also recommend treatment for a minimum of 1 to 2 months (29).
There were more AEs in the meloxicam group than in the bedinvetmab group. The most common category of AE in the meloxicam group was digestive tract disorders (diarrhoea and emesis), and it is well recognised that NSAIDs, such as meloxicam, can be associated with such AEs (10, 11, 58, 59). None of these gastrointestinal AEs were categorised as “severe,” but three were significant enough that a decision was made to withdraw the subject from the study. The rate of gastrointestinal AEs observed in this study was consistent with previous studies, which reported 12–15% of gastrointestinal AEs associated with meloxicam treatment (5, 12).
We also found a statistically significant rise in blood creatinine concentrations in the meloxicam group. NSAIDs, including meloxicam, are known to affect renal function; however, to our knowledge, a rise in creatinine associated with daily meloxicam treatment has not been reported previously. Another study examined blood biochemistry in dogs with hip osteoarthritis before and after 30 days of meloxicam treatment but did not identify any significant changes (60). None of the dogs in this study had blood creatinine levels at day 56 that exceeded the normal reference range, and none developed clinical signs of renal disease. Additionally, other related parameters, such as BUN and urine-specific gravity, did not exhibit significant changes during the study.
Assessing compliance for meloxicam in this study is challenging due to likely variations in specific gravity values amongst different generic brands. An examination of the excipients in these products revealed considerable inconsistencies between brands, which contribute to the variation in specific gravity. Nevertheless, there remains a possibility of some loss of compliance with daily oral medication administered by clients at home. Underdosing may lead to a reduction in efficacy, but conversely, it might also reduce AEs. Although this was a clinical trial with visits every 14 days, leading one to expect clients to be more vigilant regarding compliance, previous research indicates that median compliance amongst dog owners for chronic conditions can be as low as 56% (61). For chronic conditions such as OA, compliance is likely a crucial factor in providing effective pain relief and improving animal welfare.
There were only four AEs in the bedinvetmab group. Two of these were cases of lack of efficacy (LOE), compared to three such AEs in the meloxicam. One AE involved an interdigital cyst, which was likely unrelated to the test compound but interfered with mobility and the owner’s ability to use the COI effectively. As a result, the dog was withdrawn from the study. The remaining AE was polydipsia. Following the regulatory approval of bedinvetmab, pharmacovigilance data have revealed polydipsia (and associated polyuria) as a rare AE associated with bedinvetmab administration,1 which is now included in the summary of product characteristics in the United Kingdom and European Union. A rare AE is defined as occurring “1–10 animals per 10,000 animals treated.” At the time of writing, the authors understand that the pathophysiology of polydipsia associated with the administration of bedinvetmab remains unknown. In this study, the client considered the issue mild and tolerable and chose not to withdraw the dog from the study due to the observed efficacy of the product. Polydipsia and polyuria were not reported as statistically significant AEs in clinical trials of tanezumab, a similar medicine for humans (62), highlighting the species-specific nature of AEs.
A phenomenon of the current era is the emergence of social media groups linked to the launch of new medicines. Such groups can grow large but often lack rigorous analysis, context regarding the number of treated dogs, verified veterinarian oversight for pets’ overall medical conditions, and considerations for age-matched control populations. The quality of data is higher in the post-registration pharmacovigilance structures and methods of regulatory authorities such as the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in the European Union, and the Veterinary Medicines Directorate (VMD) in the United Kingdom. Nevertheless, these social media groups can have an impact (63). Anecdotally, they have reported neurological signs associated with bedinvetmab administration. No such AEs were noted in the study reported herein, but it is interesting to note that three dogs in the meloxicam group experienced neurological AEs during the study, including two with ataxia and loss of proprioception.
All dogs in the study were clinically screened for neurological disorders at the beginning, and such comorbidities were grounds for exclusion. Acute onset ataxia is recognised as a common issue in older dogs (64), as is vestibular syndrome (65). In an age-associated disease such as OA, it is important to consider suspected adverse treatment events in the context of the background event rate within the target population. It should be noted that in the overall dog population, the proportion of dogs with a history of neurologic conditions increases from less than 1% in puppies and adolescents to 12% in senior dogs, with larger breeds showing a steeper increase across age compared to dogs under 40 kg (66). The dogs in this study had an average age of over 10 years, and the older age range of dogs in studies such as this probably makes such AEs more likely; further studies are required to define the expected AE rates for various body systems in older dog populations.
Overall, from a safety perspective, this study demonstrated a lower AE rate in dogs treated with bedinvetmab (Librela, Zoetis) compared to those treated with meloxicam during a 56-day treatment period. Furthermore, the number needed to harm for meloxicam, in relation to bedinvetmab, was five. In other words, on average, five patients would need to be treated with meloxicam for one to experience harm beyond what occurs with bedinvetmab. However, it must be acknowledged that this study lasted for only 56 days, which limits the safety data conclusions. Longer-term treatment with NSAIDs compared to bedinvetmab will need further research to document any differences during extended treatment periods.
In summary, this is the first comparator clinical trial of bedinvetmab versus the NSAID meloxicam for managing pain associated with canine osteoarthritis. The results indicate that bedinvetmab performed comparably to meloxicam in effectively managing OA pain, starting on day 14. Both products demonstrated increasing efficacy over the 56-day period. Although a positive trend was observed for bedinvetmab (the bedinvetmab group exhibited a larger mean reduction in COI scores), there was no significant difference in efficacy between the two products. Both resulted in a statistically significant reduction in COI scores from baseline. This study demonstrated that bedinvetmab had a lower AE rate compared to meloxicam. These data will be valuable for clinicians and owners when making decisions regarding analgesic medications for managing canine osteoarthritis.
Data availability statement
The datasets presented in this article are not readily available because data will be held by Zoetis. Requests to access the datasets should be directed to Oliver Knesl, Oliver.knesl@zoetis.com.
Ethics statement
The animal studies were approved by Zoetis VMRD ethical review assessment committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. BL: Conceptualization, Methodology, Writing – review & editing. DB: Investigation, Writing – review & editing. RT: Investigation, Writing – review & editing. AM: Investigation, Writing – review & editing. CN: Investigation, Writing – review & editing. MW: Investigation, Writing – review & editing. KH: Investigation, Writing – review & editing. VH: Investigation, Writing – review & editing. TM: Data curation, Formal analysis, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Zoetis through a contract with Innes and Innes Partnership, an independent business partnership registered in the United Kingdom, which also managed the study.
Acknowledgments
The authors are grateful to all the clients and clinical team members who contributed to the completion of this study. Additionally, we thank Edwina Gildea for her valuable comments on the study’s design, planning, and execution.
Conflict of interest
The authors declare that this study received funding from ‘Innes and Innes Partnership’ which had a paid contract with Zoetis. The funder had the following involvement in the study: the study design was drafted by ‘Innes and Innes’ and Zoetis had input in to the study design; the final design was agreed by both parties.
JI and BL completed paid consultancy work for Zoetis in the past 2 years.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor FS declared a past co-authorship with the author JI.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1502218/full#supplementary-material
Footnotes
References
1. Anderson, KL, O'Neill, DG, Brodbelt, DC, Church, DB, Meeson, RL, Sargan, D, et al. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep. (2018) 8:8. doi: 10.1038/s41598-018-23940-z
2. Enomoto, M, de Castro, N, Hash, J, Thomson, A, Nakanishi-Hester, A, Perry, E, et al. Prevalence of radiographic appendicular osteoarthritis and associated clinical signs in young dogs. Sci Rep. (2024) 14:2827. doi: 10.1038/s41598-024-52324-9
3. Wright, A, Amodie, DM, Cernicchiaro, N, Lascelles, BDX, Pavlock, AM, Roberts, C, et al. Identification of canine osteoarthritis using an owner-reported questionnaire and treatment monitoring using functional mobility tests. J Small Anim Pract. (2022) 63:609–18. doi: 10.1111/jsap.13500
4. Innes, JF, Clayton, J, and Lascelles, BDX. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec. (2010) 166:226–30. doi: 10.1136/vr.c97
5. Walton, MB, Cowderoy, EC, Wustefeld-Janssens, B, Lascelles, BDX, and Innes, JF. Mavacoxib and meloxicam for canine osteoarthritis: a randomised clinical comparator trial. Vet Rec. (2014) 175:280. doi: 10.1136/vr.102435
6. Sanderson, RO, Beata, C, Flipo, RM, Genevois, JP, Macias, C, Tacke, S, et al. Systematic review of the management of canine osteoarthritis. Vet Rec. (2009) 164:418–24. doi: 10.1136/vr.164.14.418
7. Aragon, CL, Hofmeister, EH, and Budsberg, SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. Javma-Journal of the American Veterinary Medical Association. (2007) 230:514–21. doi: 10.2460/javma.230.4.514
8. Moreau, M, Dupuis, J, Bonneau, NH, and Desnoyers, M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. (2003) 152:323–9. doi: 10.1136/vr.152.11.323
9. Doig, PA, Purbrick, KA, Hare, JE, and McKeown, DB. Clinical efficacy and tolerance of meloxicam in dogs with chronic osteoarthritis. Canadian Veterinary Journal-Revue Veterinaire Canadienne. (2000) 41:296–300.
10. Hunt, JR, Dean, RS, Davis, GND, and Murrell, JC. An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Vet J. (2015) 206:183–90. doi: 10.1016/j.tvjl.2015.07.025
11. Mabry, K, Hill, T, and Tolbert, MK. Prevalence of gastrointestinal lesions in dogs chronically treated with nonsteroidal anti-inflammatory drugs. J Vet Intern Med. (2021) 35:853–9. doi: 10.1111/jvim.16057
12. Nell, T, Bergman, J, Hoeijmakers, M, van Laar, P, and Horspool, LJI. Comparison of vedaprofen and meloxicam in dogs with musculoskeletal pain and inflammation. J Small Anim Pract. (2002) 43:208–12. doi: 10.1111/j.1748-5827.2002.tb00059.x
13. McNamee, KE, Burleigh, A, Gompels, LL, Feldmann, M, Allen, SJ, Williams, RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. (2010) 149:386–92. doi: 10.1016/j.pain.2010.03.002
14. Vincent, TL. Peripheral pain mechanisms in osteoarthritis. Pain. (2020) 161:S138–46. doi: 10.1097/j.pain.0000000000001923
15. Shutov, LP, Warwick, CA, Shi, XY, Gnanasekaran, A, Shepherd, AJ, Mohapatra, DP, et al. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci. (2016) 36:5055–70. doi: 10.1523/JNEUROSCI.3249-15.2016
16. Enomoto, M, Mantyh, PW, Murrell, J, Innes, JF, and Lascelles, BDX. Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet Rec. (2019) 184:23. doi: 10.1136/vr.104590
17. Bannwarth, B, and Kostine, M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs. (2014) 74:619–26. doi: 10.1007/s40265-014-0208-6
18. Chang, DS, Hsu, E, Hottinger, DG, and Cohen, SP. Anti-nerve growth factor in pain management: current evidence. J Pain Res. (2016) 9:373–83. doi: 10.2147/JPR.S89061
19. Mantyh, PW, Koltzenburg, M, Mendell, LM, Tive, L, and Shelton, DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. (2011) 115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5
20. McKelvey, L, Shorten, GD, and O'Keeffe, GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem. (2013) 124:276–89. doi: 10.1111/jnc.12093
21. Seidel, MF, Wise, BL, and Lane, NE. Nerve growth factor: an update on the science and therapy. Osteoarthr Cartil. (2013) 21:1223–8. doi: 10.1016/j.joca.2013.06.004
22. Gruen, ME, Thomson, AE, Griffith, EH, Paradise, H, Gearing, DP, and Lascelles, BDX. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med. (2016) 30:1138–48. doi: 10.1111/jvim.13972
23. Lascelles, BDX, Knazovicky, D, Case, B, Freire, M, Innes, JF, Drew, AC, et al. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res. (2015) 11:11. doi: 10.1186/s12917-015-0413-x
24. Corral, MJ, Moyaert, H, Fernandes, T, Escalada, M, Tena, JKS, Walters, RR, et al. A prospective, randomised, blinded, placebo-controlled multisite clinical study of bedinvetmab, a canine monoclonal antibody targeting nerve growth factor, in dogs with osteoarthritis. Vet Anaesth Analg. (2021) 48:943–55. doi: 10.1016/j.vaa.2021.08.001
25. Michels, GM, Honsberger, NA, Walters, RR, Tena, JKS, and Cleaver, DM. A prospective, randomised, double-blind, placebo-controlled multisite, parallel-group field study in dogs with osteoarthritis conducted in the United States of America evaluating bedinvetmab, a canine anti-nerve growth factor monoclonal antibody. Vet Anaesth Analg. (2023) 50:446–58. doi: 10.1016/j.vaa.2023.06.003
26. Reid, J, Gildea, E, Davies, V, Thompson, J, and Scott, M. Measuring the effect of the anti-nerve growth factor antibodies bedinvetmab and frunevetmab on quality of life in dogs and cats with osteoarthritis using a validated health-related quality of life outcome measure: an observational real-world study. Frontiers in Veterinary Science. (2024) 11:1395360. doi: 10.3389/fvets.2024.1395360
27. Gruen, ME, Lascelles, BDX, Colleran, E, Gottlieb, A, Johnson, J, Lotsikas, P, et al. 2022 AAHA pain management guidelines for dogs and cats. J Am Anim Hosp Assoc. (2022) 58:55–76. doi: 10.5326/JAAHA-MS-7292
28. Monteiro, BP, Lascelles, BDX, Murrell, J, Robertson, S, Steagall, PVM, and Wright, B. WSAVA guidelines for the recognition, assessment and treatment of pain. J Small Anim Pract. (2022) 64:177–254. doi: 10.1111/jsap.13566
29. Cachon, T, Frykman, O, Innes, JF, Lascelles, BDX, Okumura, M, Sousa, P, et al. COAST development Group's international consensus guidelines for the treatment of canine osteoarthritis. Frontiers in Veterinary Science. (2023) 10:10. doi: 10.3389/fvets.2023.1137888
30. Brown, DC. The canine orthopedic index. Step 1: devising the items. Vet Surg. (2014) 43:232–40. doi: 10.1111/j.1532-950X.2014.12142.x
31. Brown, DC. The canine orthopedic index. Step 2: psychometric testing. Vet Surg. (2014) 43:241–6. doi: 10.1111/j.1532-950X.2014.12141.x
32. Brown, DC. The canine orthopedic index. Step 3: responsiveness testing. Vet Surg. (2014) 43:247–54. doi: 10.1111/j.1532-950X.2014.12162.x
33. FDA. What veterinarians should advise clients about pain control and nonsteroidal anti-inflammatory drugs. (2024). Available at: https://www.fda.gov/animal-veterinary/resources-you/what-veterinarians-should-advise-clients-about-pain-control-and-nonsteroidal-anti-inflammatory-drugs.
34. European Medicines Agency. Combined veterinary dictionary for drug regulatory activities (VeDDRA) list of clinical terms for reporting suspected adverse events in animals and humans to veterinary medicinal products. (2021). European Medicines Agency; 01/10/2021. Report No.: EMA/CVMP/PhVWP/10418/2009 Rev.14 corr.
35. Peterson, KD, and Keefe, TJ. Effects of meloxicam on severity of lameness and other clinical signs of osteoarthritis in dogs. Javma-Journal of the American Veterinary Medical Association. (2004) 225:1056–60. doi: 10.2460/javma.2004.225.1056
36. Lavrijsen, ICM, Heuven, HCM, Meij, BP, Theyse, LFH, Nap, RC, Leegwater, PAJ, et al. Prevalence and co-occurrence of hip dysplasia and elbow dysplasia in Dutch pure-bred dogs. Prev Vet Med. (2014) 114:114–22. doi: 10.1016/j.prevetmed.2014.02.001
37. Lavrijsen, ICM, Heuven, HCM, Voorhout, G, Meij, BP, Theyse, LFH, Leegwater, PAJ, et al. Phenotypic and genetic evaluation of elbow dysplasia in Dutch Labrador retrievers, Golden retrievers, and Bernese Mountain dogs. Vet J. (2012) 193:486–92. doi: 10.1016/j.tvjl.2012.01.001
38. Wang, S, Leroy, G, Malm, S, Lewis, T, Viklund, Å, Strandberg, E, et al. Genetic correlations of hip dysplasia scores for Golden retrievers and Labrador retrievers in France, Sweden and the UK. Vet J. (2017) 226:51–6. doi: 10.1016/j.tvjl.2017.07.006
39. Rausch-Derra, L, Huebner, M, Wofford, J, and Rhodes, L. A prospective, randomised, masked, placebo-controlled multisite clinical study of Grapiprant, an EP4 prostaglandin receptor antagonist (PRA), in dogs with osteoarthritis. J Vet Intern Med. (2016) 30:756–63. doi: 10.1111/jvim.13948
40. Brown, DC, Bell, M, and Rhodes, L. Power of treatment success definitions when the canine brief pain inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res. (2013) 74:1467–73. doi: 10.2460/ajvr.74.12.1467
41. Radke, H, Joeris, A, and Chen, M. Evidence-based evaluation of owner-reported outcome measures for canine orthopedic care - a COSMIN evaluation of 6 instruments. Vet Surg. (2022) 51:244–53. doi: 10.1111/vsu.13753
42. Alves, JC, and Innes, JF. Minimal clinically-important differences for the "Liverpool osteoarthritis in dogs" (LOAD) and the "canine orthopedic index" (COI) in dogs with osteoarthritis. PLoS One. (2023) 18:e0291881. doi: 10.1371/journal.pone.0291881
43. Innes, JF, Morton, MA, and Lascelles, BDX. Minimal clinically-important differences for the 'Liverpool osteoarthritis in Dogs' (LOAD) and the 'Canine orthopedic Index' (COI) client-reported outcomes measures. PLoS One. (2023) 18:e0280912. doi: 10.1371/journal.pone.0280912
44. Alves, JC, Santos, A, Jorge, P, Lavrador, C, and Carreira, LM. Evaluation of the thermographic response of the lumbar region in dogs with bilateral hip osteoarthritis. J Therm Biol. (2023) 115:103610. doi: 10.1016/j.jtherbio.2023.103610
45. Alves, JC, Santos, A, Jorge, P, and Lafuente, P. A multiple-session mesotherapy protocol for the management of hip osteoarthritis in police working dogs. Am J Vet Res. (2023) 84:1–8. doi: 10.2460/ajvr.22.08.0132
46. Alves, JC, Santos, A, and Carreira, LM. A preliminary report on the combined effect of intra-articular platelet-rich plasma injections and Photobiomodulation in canine osteoarthritis. Animals. (2023) 13:3247. doi: 10.3390/ani13203247
47. Alves, JCA, Jorge, PIF, and Dos Santos, A. A survey on the orthopedic and functional assessment in a Portuguese population of police working dogs. BMC Vet Res. (2022) 18:116. doi: 10.1186/s12917-022-03221-8
48. Alves, JC, Santos, A, Jorge, P, and Carreira, LM. A randomised double-blinded controlled trial on the effects of photobiomodulation therapy in dogs with osteoarthritis. Am J Vet Res. (2022) 83:ajvr.22.03.0036. doi: 10.2460/ajvr.22.03.0036
49. Alves, JC, Santos, A, Jorge, P, and Carreira, LM. A first report on the efficacy of a single intra-articular administration of blood cell secretome, triamcinolone acetonide, and the combination of both in dogs with osteoarthritis. BMC Vet Res. (2022) 18:309. doi: 10.1186/s12917-022-03413-2
50. Alves, JC, Santos, A, Jorge, P, and Carreira, LM. A comparison of intra-articular blood cell Secretome and blood cell Secretome with triamcinolone Acetonide in dogs with osteoarthritis: a crossover study. Animals. (2022) 12:3358. doi: 10.3390/ani12233358
51. Alves, JC, Santos, A, and Jorge, P. Platelet-rich plasma therapy in dogs with bilateral hip osteoarthritis. BMC Vet Res. (2021) 17:207. doi: 10.1186/s12917-021-02913-x
52. Alves, JC, Santos, A, Jorge, P, Lavrador, C, and Carreira, LM. Clinical and diagnostic imaging findings in police working dogs referred for hip osteoarthritis. BMC Vet Res. (2020) 16:425. doi: 10.1186/s12917-020-02647-2
53. Alves, JC, Santos, A, Jorge, P, Lavrador, C, and Carreira, LM. A report on the use of a single intra-articular administration of autologous platelet therapy in a naturally occurring canine osteoarthritis model-a preliminary study. BMC Musculoskelet Disord. (2020) 21:127. doi: 10.1186/s12891-020-3140-9
54. Hercock, CA, Pinchbeck, G, Giejda, A, Clegg, PD, and Innes, JF. Validation of a client-based clinical metrology instrument for the evaluation of canine elbow osteoarthritis. J Small Anim Pract. (2009) 50:266–71. doi: 10.1111/j.1748-5827.2009.00765.x
55. Walton, MB, Cowderoy, E, Lascelles, D, and Innes, JF. Evaluation of construct and criterion validity for the 'Liverpool osteoarthritis in Dogs' (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS One. (2013) 8:e58125. doi: 10.1371/journal.pone.0058125
56. Brown, DC, Boston, RC, and Farrar, JT. Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. J Vet Intern Med. (2013) 27:22–30. doi: 10.1111/jvim.12004
57. Brown, DC, Boston, RC, Coyne, JC, and Farrar, JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. Javma-Journal of the American Veterinary Medical Association. (2008) 233:1278–83. doi: 10.2460/javma.233.8.1278
58. Cozzi, EM, and Spensley, MS. Multicenter randomised prospective clinical evaluation of meloxicam administered via transmucosal oral spray in client-owned dogs. J Vet Pharmacol Ther. (2013) 36:609–16. doi: 10.1111/jvp.12050
59. Monteiro-Steagall, BP, Steagall, PVM, and Lascelles, BDX. Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs. J Vet Intern Med. (2013) 27:1011–9. doi: 10.1111/jvim.12127
60. Molina, DV, Alzate, VD, Ruíz, BJ, Urrea, AM, and Tobón, JJ. Analgesic effect and side effects of celecoxib and meloxicam in canine hip osteoarthritis. Revista Mvz Cordoba. (2014) 19:4289–300. doi: 10.21897/rmvz.91
61. Booth, S, Meller, S, Packer, RM, Farquhar, R, Maddison, JE, and Volk, HA. Owner compliance in canine epilepsy. Vet Rec. (2021) 188:262–9. doi: 10.1002/vetr.16
62. Gao, YJ, Hu, ZX, Huang, Y, Liu, WJ, and Ren, CL. Efficacy and safety of anti-nerve growth factor antibody therapy for hip and knee osteoarthritis: a Meta-analysis. Orthop J Sports Med. (2022) 10:23259671221088590. doi: 10.1177/23259671221088590
63. Calfas, J. What killed their pets? Owners blame meds, but vets Aren’t sure. Wall Street J. (2024) April 12, 2024 5:30 am ET
64. Cherubini, GB, Lowrie, M, and Anderson, J. Pelvic limb ataxia in the older dog - 1. Assessment and non-painful conditions In Practice. (2008) 30:386–91. doi: 10.1136/inpract.30.7.386
65. Radulescu, SM, Humm, K, Eramanis, LM, Volk, HA, Church, DB, Brodbelt, D, et al. Vestibular disease in dogs under UK primary veterinary care: epidemiology and clinical management. J Vet Intern Med. (2020) 34:1993–2004. doi: 10.1111/jvim.15869
Keywords: bedinvetmab, meloxicam, canine orthopaedic index, canine osteoarthritis, degenerative joint disease, monoclonal antibody, pain management
Citation: Innes JF, Lascelles BDX, Bell D, Tulloch R, McVey A, Northcott C, Welbourn M, Higgins K, Horakova V and Maddox TW (2025) A randomised, parallel-group clinical trial comparing bedinvetmab to meloxicam for the management of canine osteoarthritis. Front. Vet. Sci. 12:1502218. doi: 10.3389/fvets.2025.1502218
Edited by:
Francesco Staffieri, University of Bari Aldo Moro, ItalyReviewed by:
Claudia Interlandi, University of Messina, ItalyIoannis Savvas, Aristotle University of Thessaloniki, Greece
Copyright © 2025 Innes, Lascelles, Bell, Tulloch, McVey, Northcott, Welbourn, Higgins, Horakova and Maddox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John F. Innes, john.innes@movementvets.co.uk
 John F. Innes
John F. Innes